| disease | Acute Arterial Embolism |
Embolism occurs when a detached thrombus blocks the stirred pulse, leading to an acute vascular disease. When peripheral stirred pulse embolism occurs, the affected limb exhibits pain, pallor, coldness, numbness, motor dysfunction, and weakened or absent stirred pulse. The incidence of peripheral stirred pulse embolism has been gradually increasing. According to a recent report from Henry Ford Hospital, the rate of stirred pulse embolism among hospitalized patients was 23.1 per 100,000 from 1954 to 1965, which increased to 54.5 per 100,000 from 1964 to 1979. This reflects the growing elderly population, prolonged lifespans of cardiac patients, and the broader application of invasive vascular techniques.
bubble_chart Etiology
Embolism caused by thrombosis often occurs in patients with heart blood vessel disease. The sources of embolism include the following aspects.
(1) Cardiogenic Many reports indicate that the most common disease cause of peripheral stirred pulse embolism is cardiogenic. In 1977, Fogarty reported 338 cases of stirred pulse embolism, with 94% originating from heart disease, 77% of which were accompanied by atrial fibrillation. In recent years, the nature and corresponding incidence of cardiogenic sources have changed. Rheumatic heart disease no longer holds the absolute dominance it once did, as antagonism stirred pulse sclerosis and myocardial infarction have come to play more significant roles. Stirred pulse sclerotic coronary stirred pulse heart disease, including myocardial infarction, atrial fibrillation, congestive heart failure, and ventricular wall stirred pulse aneurysm, accounts for about 60%, while rheumatic heart disease accounts for 20%. Both rheumatic heart disease and coronary stirred pulse heart disease involve thrombus formation in the left heart. In rheumatic heart disease, especially with mitral stenosis, slowed blood flow in the atrium combined with inner membrane wind-warmth changes makes it easier for fibrin in the blood to adhere to the atrial wall and form a thrombus. In coronary stirred pulse heart disease, particularly during myocardial infarction, when the left ventricle is enlarged and contraction lacks strength, blood cannot be fully expelled, making thrombus formation more likely.
(2) Vascular Origin Embolisms formed by stirred pulse aneurysm and stirred pulse sclerotic atheromatous material have been increasingly reported in recent years. Large embolisms can originate from mixtures of large stirred pulse atheromatous material, thrombi, and cholesterol crystals that break off and enter the stirred pulse circulation. Small embolisms may result from the release of cholesterol crystals or the detachment of ulcerative stirred pulse hard dissipating ecchymosis points.(3) Iatrogenic In recent years, the widespread use of artificial heart valve membrane replacements, artificial blood vessel transplants, pacemaker implantation, stirred pulse angiography, arteriovenous fistulas for hemodialysis, indwelling stirred pulse catheters, and large stirred pulse counterpulsation balloon catheters has increased the potential for stirred pulse embolism.
Zhongshan Hospital in Shanghai summarized 43 cases and 50 instances of stirred pulse embolism treated between December 1963 and December 1984. The sources of stirred pulse emboli are shown in Table 41-1. Among them, 18 cases (42.4%) involved apoplexy rheumatic heart disease with atrial fibrillation, and 6 cases (13.4%) involved atrial fibrillation after rheumatic heart disease valve replacement. Thus, rheumatic heart disease accounted for 24 cases (4.6%), which differs from the nature of cardiogenic sources reported in foreign literature.
Table 41-1 Sources of Emboli
| Source of Emboli | Number of Cases | % |
| Rheumatic Heart Disease with Atrial Fibrillation | 18 | 42.4 |
| Atrial Fibrillation After Rheumatic Heart Disease Valve Replacement | 6 | 13.4 |
| Coronary Heart Disease with Atrial Fibrillation | 2 | 4.6 |
| Hypertensive Heart Disease with Atrial Fibrillation | 4 | 9.2 |
| Hyperthyroidism with Atrial Fibrillation | 1 | 2.3 |
| Atrial Fibrillation of Unknown Cause | 1 | 2.3 |
| After Stirred Pulse Aneurysm Removal and Blood Vessel Transplant | 1 | 2.3 |
| After Stirred Pulse Retrograde Catheterization | 1 | 2.3 |
| Subacute bacterial endocarditis | 1 | 2.3 |
| Eisenmenger syndrome | 1 | 2.3 |
| Unknown cause | 1 | 2.3 |
At the bifurcation of the stirred pulse, the lumen suddenly narrows, combined with the saddle-shaped anatomical formation, leading to emboli almost exclusively occurring at the stirred pulse bifurcation and branch openings. If the patient previously had stirred pulse sclerosis or sexually transmitted disease causing stenosis, embolisms mostly occur at the stenotic lesions. Fogarty et al. reported 338 cases of stirred pulse embolism, with the most common sites being the terminal abdominal stirred pulse, iliac stirred pulse, femoral stirred pulse, and popliteal stirred pulse, totaling 302 cases.
After embolism occurs, the stirred pulse lumen may be partially or completely obstructed, leading to the following pathophysiological changes: ① Stirred pulse spasm: The embolism stimulates the stirred pulse wall nerves, triggering reflex vasoconstriction via the sympathetic vasomotor center, causing intense spasms in the distal vessels and adjacent collateral stirred pulses. Large amounts of aggregated platelets within the thrombus release histamine and serotonin, exacerbating stirred pulse spasm. The more severe the spasm, the worse the ischemia and the higher the risk of gangrene. ② Secondary thrombosis: Spasms can also affect the stirred pulse's own nutrient vessels, leading to vascular wall blood flow disorders. Damage to vascular endothelial cells, thickening and rupture of the internal elastic lamina are all significant factors contributing to secondary thrombosis. The drop in pressure in the distal stirred pulse segment causes slowed blood flow, lumen collapse, and the release of coagulants during thrombus contraction, along with adenosine diphosphate released by red blood cells, white blood cells, and platelets, accelerating blood coagulation. Muscle and nerve tissues produce small amounts of prostaglandin E, which inhibits collagen fibers, prothrombin, adrenaline, and adenosine diphosphate, all of which can induce platelet aggregation. After stirred pulse embolism, ischemia in adjacent tissues reduces prostaglandin production, increasing the levels of these substances and accelerating thrombus propagation. ③ Changes in the affected limb: Tissue hypoxia leads to cell necrosis, with different cells having varying sensitivities to hypoxia and oxygen consumption rates. For example, the retina's oxygen consumption rate is four times higher than that of skin cells. Peripheral nerves and muscles have higher oxygen consumption rates than skin. Generally, within 15–30 minutes of stirred pulse embolism, symptoms of nerve ischemia appear, starting with sensory decline and paresthesia, followed by muscle group paralysis. If blood flow is restored within 30–60 minutes, the ischemic limb can still recover; otherwise, severe changes occur. Muscles die within 6–12 hours, nerves are destroyed after 12–20 hours, and skin necrosis occurs within 24–48 hours. ④ Cardiac effects of embolism: Most patients have heart blood vessel system diseases, and stirred pulse embolism more or less increases cardiac workload. Generally, the larger the embolized stirred pulse, the more pronounced the obstruction and spasm, and the greater the impact on the heart. ⑤ Systemic metabolic effects of embolism: After embolism occurs, extensive tissue involvement and rapid blood flow restoration post-embolectomy allow metabolic products from necrotic tissues to quickly enter systemic circulation, leading to significant metabolic changes in a short period, clinically termed myopathic-nephrotic-metabolic syndrome. Fischer Fogarty has studied venous blood composition and blood generation and transformation changes during limb ischemia. Venous blood oxygen levels drop, while carbon dioxide binding capacity, lactate, phosphorus, creatinine phosphokinase (CPK), LOH, and SGOT enzymes rise, along with rhabdomyolysis. When limb blood flow is restored, abdominal mass substances in the veins are immediately released into circulation. Haimovici has studied the myopathic-nephrotic-metabolic syndrome, noting that 1/3 of peripheral stirred pulse embolism deaths are caused by post-reperfusion effects. The myopathic-nephrotic-metabolic syndrome is most likely to occur in patients with severe pain, edema, and muscle or joint stiffness.
bubble_chart Clinical ManifestationsAcute arterial embolism occurs when there is occlusion without collateral circulation compensation, leading to rapid progression of the condition. The typical symptoms include pain, pallor, coldness, numbness, motor dysfunction, and weakened or absent arterial pulsation. The severity of symptoms depends on the location and extent of the embolism, the degree of secondary thrombosis, pre-existing arterial stenosis due to arteriosclerosis, and the status of collateral circulation. 1. **Pain**: Pain is often the earliest symptom, gradually extending distally. About 20% of patients experience numbness as the first symptom, with pain being less noticeable. 2. **Skin color and temperature changes**: Impaired limb circulation causes the venous plexus under the skin to empty first, resulting in a waxy pallor. If small amounts of blood remain in the vessels, scattered purplish macules may appear. Superficial veins collapse, capillary refill is slow, and the calf muscles may feel dough-like. With worsening ischemia, muscles may stiffen, and the affected limb's temperature drops, most noticeably in the distal part. The actual level of temperature change is typically one joint below the embolism site. For example: - Aortic bifurcation embolism affects the thighs and buttocks. - Common iliac artery embolism affects the lower thighs. - Common femoral artery embolism affects the mid-thigh. - Popliteal artery embolism affects the lower legs. 3. **Weakened or absent arterial pulsation**: Proximal pulses may be stronger, but transmitted pulsations from blood flow may be mistaken for distal pulses. 4. **Numbness and motor dysfunction**: The distal limb may exhibit stocking-like sensory loss due to peripheral nerve ischemia. Proximal areas may show hypoesthesia or hyperesthesia. The limb may also experience tingling, muscle weakness, or even paralysis, leading to varying degrees of foot or wrist drop.
bubble_chart Auxiliary Examination
1. Skin Temperature Measurement: It can accurately determine the boundary between normal and decreased skin temperature, thereby inferring the location of the embolism.
2. Ultrasound Examination: Doppler ultrasound can assess the blood flow of stirred pulse, providing more precise localization of the embolism. It also establishes a baseline for insufficient blood supply, facilitating pre- and post-operative comparisons to evaluate vascular reconstruction and monitor vascular patency.
3. Stirred Pulse Angiography: Angiography is the most accurate method for embolism localization. Most patients can be diagnosed based on clinical symptoms, signs, and Doppler ultrasound. Stirred pulse angiography is only performed when there is diagnostic uncertainty or when post-thrombectomy evaluation of stirred pulse patency is necessary.
After confirming the diagnosis, additional tests such as chest X-rays, electrocardiograms, cardiac X-rays, and echocardiography should be conducted to check for arrhythmias or recent myocardial infarction. This helps further identify the cause of stirred pulse embolism, enabling timely intervention and control of the disease cause.
Sudden onset of limb pain accompanied by acute arterial ischemia and disappearance of the corresponding arterial pulse generally confirms the diagnosis.
bubble_chart Treatment Measures
Peripheral arterial embolism, the timing of treatment is closely related to the survival of the limb. Specific methods are divided into surgical treatment and non-surgical treatment.
(1) Surgical treatment
1. Indications for embolectomy: The optimal time for surgery is considered to be within 12 hours after onset. If the limb tissue remains viable, embolectomy in the advanced stage can still be successful. Because the intima of the affected artery is uninjured, the distal artery was patent before the embolism, and anticoagulant therapy was administered in advance, these factors are all conducive to complete removal of the embolism and secondary thrombosis, restoring arterial patency. Of course, limb gangrene is a contraindication for embolectomy. Haimovici studied a series of untreated peripheral arterial embolisms with naturally restored circulation and classified them into four grades. Grade I, grade II ischemia, with early restoration of arterial pulsation, termed anischemic embolism, accounting for 29.5%. Grade II, severe ischemia with partial restoration of arterial pulsation in the advanced stage, termed chronic post-embolic ischemia, accounting for 22.2%. Grade III, severe ischemia leading to varying degrees of gangrene often accompanied by metabolic complications, accounting for 28%. Grade IV, the most severe ischemia, with fatal outcomes, patients have advanced heart failure or visceral arterial embolism.
2. Preoperative preparation: Take various measures to correct the patient's systemic condition and cardiac function, and adopt anticoagulant and antiplatelet therapies. Heparin is the anticoagulant of choice. Administer 50mg intravenously preoperatively and another 20–30mg during surgery. Dextran is the antiplatelet drug of choice, and intravenous infusion can be started preoperatively.
3. Anesthesia and intraoperative monitoring: Most patients can undergo embolectomy using a Fogarty balloon catheter under local anesthesia, but those requiring exposure of the groin, thigh, or popliteal fossa need epidural anesthesia. Monitoring of ECG, blood pressure, and blood gases is very helpful.
4. Surgical technique: Since the adoption of the Fogarty balloon catheter for embolectomy, the surgical method has been greatly simplified. The catheter can reach vessels in various locations, reducing restricted areas, but in some cases, direct exposure and arterial incision for embolectomy are still necessary.
5. Procedure: ① Position: For lower limbs, adopt a head-up, foot-down position, with the limb placed below the level of the heart to facilitate blood supply. ② Skin preparation: For lower limb embolectomy, include the groin and the entire limb. For upper limb embolectomy, include the chest and the entire upper limb. ③ Incision: Different incisions should be made depending on the affected site (Figure 1).
Figure 1 Embolectomy at different arterial sites
⑴ Axillary artery incision ⑵ Brachial artery incision ⑶ Rectus abdominis incision ⑷ Femoral artery incision ⑸ Lower thigh incision ⑹ Popliteal artery incision
6. Femoral embolectomy. The incision should be long enough, with a longitudinal incision made in the groin to expose the common, deep, and superficial femoral arteries. At the site of femoral artery embolism, the artery appears fusiformly dilated and slightly bluish. The proximal artery pulsates strongly, but the distal pulsation is absent. Palpating the transmitted pulsation should never be mistaken for an embolism in a more proximal artery. Gentle palpation can determine the extent of the embolism and thrombus and assess whether the arterial wall is normal. After incising the arterial sheath, the common, deep, and superficial femoral arteries are freed and looped with plastic tubes to prevent the embolism from moving into the superficial or deep femoral artery. A longitudinal incision of 1.0–1.5 cm is made in the common femoral artery down to below the deep femoral artery. The embolus will protrude spontaneously into the lumen. First, the tail of the embolus is removed, followed by slowly extracting the head of the embolus with a nasal speculum (Figure 2). An appropriately sized Fogarty catheter is inserted into the superficial femoral artery. If the patient does not have arterial atherosclerosis, the catheter can easily reach the tibial artery. After inflating the balloon, the catheter is slowly withdrawn. Once there is significant backflow from the superficial femoral artery, a 4F catheter is inserted into the deep femoral artery to remove emboli from each branch vessel. Then, a 6F catheter is inserted and withdrawn to ensure complete removal of the embolus, with visible spurting blood from the proximal artery and significant backflow from the distal artery. The artery is then flushed with 0.5% heparin solution, clamped, and repaired with sutures. If stenosis is possible after suturing, a venous patch may be needed. Finally, it is essential to confirm the patency of the reconstructed vessel, with strong pulsation in the proximal artery. Although backflow from the artery is a meaningful sign of distal artery patency, it does not rule out the possibility of residual emboli, as the backflow may come from major collateral circulation. Therefore, it is crucial to confirm the patency of the popliteal and dorsalis pedis arteries at the end of the procedure. If any doubt arises, it is best to perform angiography immediately on the operating table. If residual emboli are detected in the distal segment, an incision can be made in the lower third of the thigh and the medial popliteal region to expose the popliteal artery and its branches. The popliteal artery and the anterior and posterior tibial arteries are controlled with plastic tubes. A transverse incision is made in the popliteal artery, and F2 or F3 Fogarty catheters are inserted into the anterior and posterior tibial arteries to remove the emboli.
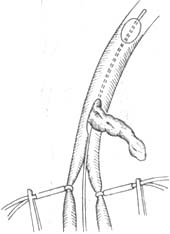
Figure 2. Iliofemoral Embolectomy Using Fogarty Catheter
7. Aortic Saddle Embolectomy
(1) Retrograde Femoral Artery Embolectomy: Disinfect the abdominal and bilateral lower limb skin, make incisions on both femoral regions to expose the common, superficial, and deep femoral arteries, and loop them with plastic tubes. Make an incision on the common femoral artery of the more ischemic side, and clamp or block the contralateral common femoral artery with a plastic tube. First, use an appropriately sized Fogarty catheter (4F–5F) to remove the embolus from the superficial femoral artery, inspect the deep femoral artery, and flush with heparin solution after obtaining good arterial backflow. Insert the balloon catheter above the renal artery, inflate the balloon with saline until resistance is felt. When pulling the catheter from the aorta to the iliac artery, slightly deflate the balloon to match the diameter of the iliac artery. Pull the balloon catheter out through the common femoral artery incision to remove the embolus. This procedure can be repeated several times until pulsatile flow is restored. After achieving good blood flow on one side, attention should turn to the contralateral side. The contralateral side should exhibit good pulsation; if thrombus is suspected, remove it using the same method. Close the femoral artery routinely. After arterial closure, assess distal arterial pulsation, skin color, and superficial venous filling. Intraoperative Doppler can also be used to measure blood flow. If arterial pulsation is unsatisfactory, perform intraoperative arteriography and re-explore if flow is compromised.
(2) Direct Aortic Embolectomy: This method is rarely used today. When preexisting arteriosclerotic stenosis makes retrograde femoral embolectomy impossible, direct exposure of the aortic bifurcation is required. Make a left paramedian incision from above the umbilicus to the pubic symphysis. Retract the small intestine to one side, and incise the posterior peritoneum along the aortic pulsation down to the pelvis. Mobilize the aorta and bilateral iliac arteries below the inferior mesenteric artery, looping them with plastic tubes. Make an incision on one common iliac artery to remove the embolus and thrombus from the aortic saddle. Then use a Fogarty balloon catheter for distal arterial embolectomy. If emboli are suspected on the other side, incise the contralateral iliac artery and perform embolectomy as described.
(3) Retroperitoneal Approach for Aortic Saddle Embolectomy: In thin patients, the left retroperitoneal approach to expose the aortic saddle has many advantages and carries less surgical risk. In obese patients, this approach makes exposure of the right iliac artery difficult. If the patient has a history of chronic ischemia due to distal arteriosclerosis, this approach is beneficial for performing lumbar sympathectomy.
8. Upper Limb Arterial Embolectomy: The incidence of upper limb arterial embolism is relatively low, accounting for 16–32% of peripheral arterial embolisms. The brachial artery is the most commonly affected, suggesting that most upper limb emboli are relatively small. For upper limb embolectomy, local or brachial plexus anesthesia is preferred. Skin preparation should include the entire upper limb and the ipsilateral anterior chest wall. Whether the embolus is in the axillary, brachial, or other arteries, it can be removed by antegrade or retrograde insertion of a Fogarty balloon catheter through the axillary or brachial artery.
9. Postoperative Management: ① Continue treating cardiac disease to restore normal rhythm. ② After revascularization of the ischemic limb, metabolic changes may rapidly affect the whole body, primarily manifesting as acidosis, hyperkalemia, and elevated levels of skeletal muscle enzymes (LDH, SGOT, CPK), which must be corrected at all levels. ③ Anticoagulation therapy: After peripheral arterial embolectomy, anticoagulation therapy is necessary. Use heparin 0.8–1.0 mg/kg, injected subcutaneously into the abdominal fat layer every 12 hours for one week, overlapping with warfarin starting on the 6th day for 2 weeks.
10. Results of Embolectomy Many factors influence the outcomes of embolectomy. Blaisdell reviewed 35 publications. Among them, 14 reported mortality rates of 15–24%, 10 reported 25–29%, and 11 reported 30–48%. The limb salvage rate was 63%, with an average mortality rate of 38%. The primary causes of death were congestive heart failure and acute myocardial infarction, followed by pulmonary embolism. Other causes included shock, mesenteric vascular embolism, and hepatic coma. Recent reports have also mentioned metabolic and renal complications. Shanghai Zhongshan Hospital summarized 43 cases of peripheral arterial embolism treated between December 1963 and December 1984, with a mortality rate of 27.9%, which is consistent with the aforementioned foreign literature. Notably, one patient at the hospital underwent aortic saddle embolectomy. Although femoral pulses were restored bilaterally postoperatively, the patient developed renal failure, underwent hemodialysis unsuccessfully, and died.
Advanced stage thrombectomy, which refers to surgery performed more than 1 to several days later. Haiwexic once reported performing surgery 22 hours to 21 days after stirred pulse embolism, with a vascular patency rate of 64.3%.
11. Complications of thrombectomy using a balloon catheter The use of a balloon catheter has many advantages but also carries potential risks. Possible complications include: ① The catheter puncturing the stirred pulse wall, causing bleeding; ② Separation of the stirred pulse membrane, leading to ulcers and secondary thrombosis; ③ Tearing of the stirred pulse hard dissipating ecchymosis; ④ Catheter breakage, leaving it lodged in the stirred pulse cavity; ⑤ Loosening of the thrombus, causing it to enter distal stirred pulse branches; ⑥ The catheter puncturing arteries and veins, resulting in arteriovenous fistula.
(2) Non-surgical treatment Applicable to: ① Embolism in the popliteal stirred pulse branches and brachial stirred pulse branches; ② Patients whose condition cannot tolerate surgery; ③ Cases where the limb has already developed gangrene and is unsuitable for thrombectomy. Non-surgical treatments include relieving stirred pulse spasms, establishing collateral circulation, preventing thrombus extension, and deep thrombosis.
1. General management Closely monitor the patient’s vital signs and the condition of the affected limb, and maintain detailed records. Position the affected limb below the level of the heart, typically at a 15º downward angle, to facilitate blood flow into the limb. Maintain room temperature at around 25ºC. Avoid local heat application, as it may increase tissue metabolism and worsen ischemia and hypoxia. Local cold compresses or cooling may cause vasoconstriction and reduce blood supply, and are therefore contraindicated.
2. Preventing thrombus extension Includes anticoagulation and antiplatelet therapies.
(1) Among various anticoagulants, heparin is the only effective and reliable drug, especially during the acute phase of embolism. Dicoumarol and other prothrombin inhibitors act too slowly and are unsuitable for emergency use.
Method of heparin administration: It is best to inject into the stirred pulse proximal to the embolism where pulsation is present. Use a 0.5% heparin solution, 10ml per dose, once every 24 hours. If heparin cannot be administered via the stirred pulse, switch to intravenous injection, 50mg per dose, 2–3 times daily.
(2) Antiplatelet therapy: Low-molecular-weight dextran not only expands volume and reduces blood viscosity but also has anti-aggregation effects and alters the electrical potential of the vascular membrane. Administer 500ml once daily. Aspirin and dipyridamole may also be used as adjunctive treatments.
(3) Thrombolytic therapy: Fibrinolytic drugs such as streptokinase or urokinase can dissolve fresh thrombi. In the U.S., they are used to treat venous and pulmonary stirred pulse embolism. The best results are generally seen for thrombi within 3 days of onset, while efficacy diminishes after 7 days. The optimal route of administration is direct puncture or catheter injection into the stirred pulse cavity proximal to the embolism. Intravenous drip may also be used.
3. Treatment to relieve vascular spasms During the acute phase of stirred pulse embolism, the following treatments may be selected: ① Intravenous drip of 0.1% procaine, 500–1000ml daily, to alleviate vascular spasms. ② Vasodilators such as papaverine (30–60mg) injected directly into the stirred pulse cavity proximal to the embolism, or administered via intramuscular injection or intravenous drip. Prostaglandins at appropriate doses not only inhibit platelet aggregation but also have vasodilatory effects. It is important to note that some authors report vasodilators should only be used for stirred pulse blood supply insufficiency. Their use in acute stirred pulse embolism and thrombotic stirred pulse occlusion may be harmful. While vasodilators may relieve vascular spasms, they may also divert blood flow from the affected area to normal vascular beds, exacerbating ischemic symptoms. They may also cause the thrombus to extend into previously spasmodic stirred pulse branches.
Sympathetic nerve block: Sympathetic nerve block is an effective measure to relieve spasms in the stirred pulse, acting on the collateral stirred pulse. Experience has shown that the clinical response to sympathetic nerve block is favorable, even when the pulsation of the main stirred pulse has not yet recovered. This not only alleviates pain but also rapidly transforms limbs that were previously cold, pale, or cyanotic into warm and pinkish states. For lower limb stirred pulse embolism, lumbar sympathetic nerve block can be performed, while for the upper limb, stellate ganglion block is the appropriate approach.
(3) Iatrogenic arterial embolism As mentioned earlier, all interventional vascular and cardiac diagnostic and therapeutic procedures may cause iatrogenic arterial embolism.
During arterial catheterization, hard calcified plaques and mural thrombi in the artery can become dislodged, fragmented, and embolized to distal arteries due to mechanical contact with puncture needles, guidewires, or catheters. Symptoms depend on the size of the dislodged material and the site of embolism. If guidewires or catheters fracture, the broken parts may travel with blood flow to distal arteries of similar caliber or bifurcations, causing embolism. In recent years, the metal tips of laser arterial catheters used clinically may occasionally detach from the catheter body and embolize distal arteries.
Sometimes, the fractured tip of a right heart catheter may pass through an atrial or ventricular septal defect into the left heart and then enter the systemic circulation, causing embolism in certain locations.
Thrombi within an arterial aneurysm may be dislodged by surgical manipulation, leading to distal arterial embolism. During artificial vascular graft placement, sutures and needles may dislodge patches from the host artery, resulting in embolism.
After artificial heart valve replacement, especially when complicated by acute bacterial endocarditis, arterial embolism is highly likely. In such cases, embolism often involves multiple organs and limbs, resulting in high mortality.
The management principles for iatrogenic arterial embolism are the same as those for arterial embolism mentioned earlier, namely prompt and complete removal of the embolus. The key to management is early detection. Before concluding arterial catheterization or surgery, it is essential to carefully inspect all instruments for integrity and ensure that distal arterial blood return is normal or restored to pre-incision levels. Preoperative, intraoperative, and postoperative assessment of arterial pulsations or Doppler ultrasound, as well as plain X-rays or even arteriography, are effective measures for early detection of iatrogenic arterial embolism.
In addition to the more common iatrogenic arterial embolisms mentioned above, patients receiving warfarin therapy may occasionally develop cholesterol microemboli, causing "blue toe syndrome." Cholesterol microemboli can widely embolize the central retinal artery, heart, brain, liver, pancreas, spleen, kidneys, and other organs, leading to corresponding functional impairments or even death. The prognosis depends on the extent of embolism, and there is currently no effective treatment.
(1) Acute stirred pulse thrombosis Clinically, it is very difficult to differentiate between acute stirred pulse embolism and secondary thrombosis caused by stirred pulse atherosclerosis, but distinguishing the two is crucial. Thrombectomy using the balloon catheter method is relatively safe and effective. However, thrombectomy often fails and may even expand the scope of obstruction. Stirred pulse thrombosis presents with long-term symptoms of insufficient blood supply, such as numbness, fear of cold, and intermittent claudication. Examination reveals skin, nail, and muscle atrophy. The onset is not as abrupt as stirred pulse embolism and is often preceded by a period of vascular insufficiency. Stirred pulse angiography shows rough, uneven, or twisted vessel walls, stenosis, and segmental obstruction, with numerous collateral circulations appearing twisted or spiral-shaped. Noticing these features aids in differential diagnosis.
(2) Acute deep vein thrombosis Acute iliofemoral thrombophlebitis or phlegmasia cerulea dolens may cause reflexive spasm of the stirred pulse, leading to weakened or absent distal stirred pulse pulsations, decreased skin temperature, pale skin, and limb edema, which can be misdiagnosed as stirred pulse embolism. Edema is often a manifestation of severe stirred pulse insufficiency in advanced stages, with significant skin and muscle ischemia occurring first. However, in most cases of thrombophlebitis, severe edema occurs before skin necrosis. Concurrent superficial varicose veins and cyanotic skin discoloration help differentiate it from stirred pulse embolism.
(3) Stirred pulse intimal dissection Stirred pulse intimal dissection can create a false sinus within the lumen, compressing the stirred pulse cavity and potentially causing distal stirred pulse embolic obstruction. These patients often have a history of chest or back pain, long-term hypertension, audible murmurs on auscultation, and widened mediastinum on chest X-rays, which aid in diagnosis.
Additionally, peripheral stirred pulse aneurysm thrombosis, popliteal entrapment syndrome, and ergot intoxication may also cause intermittent claudication and severe ischemic symptoms, requiring careful differentiation.




