| disease | Dysfunctional Uterine Bleeding |
| alias | Functional Blood, Dysfunctional Uterine Bleeding, DUB |
Dysfunctional uterine bleeding (DUB), referred to as functional bleeding, is abnormal uterine bleeding characterized by menstrual disorders caused by dysfunction of the HPOU axis rather than organic sexually transmitted diseases of the reproductive tract.
bubble_chart Etiology
- Systemic factors: including adverse psychological trauma, stress, malnutrition, endocrine and metabolic disorders such as iron deficiency, anemia, aplastic anemia, hematologic and hemorrhagic diseases, diabetes, thyroid and adrenal disorders.
- Dysfunction of the HPO axis: including disturbances in the rhythm of reproductive hormone release, feedback dysfunction, and ovulatory and luteal phase defects.
- Uterine and endometrial factors: including spiral arteriole disturbances, structural and functional abnormalities of the microcirculatory vascular bed, dysfunction of endometrial steroid receptors and lysosomes, local coagulation abnormalities, and imbalances in prostaglandin TXA2 and PGI2 secretion.
- Iatrogenic factors: including steroidal contraceptives and intrauterine devices interfering with normal HPO axis function. Certain medications for systemic diseases (especially those affecting the nervous and psychiatric systems) can affect normal menstrual function through neuroendocrine mechanisms.
Normal menstruation cycle is a biological clock phenomenon influenced by internal and external environmental factors and regulated by neuroendocrine systems, ensuring that female reproductive physiology and endocrine functions adhere to strict biological rhythms, including distinct circadian rhythm, lunar rhythm, and seasonal rhythms. Any factors interfering with the neuroendocrine regulation of menstruation can lead to menstruation disorders and abnormal uterus bleeding.
1. **Sex Hormone Secretion Dysregulation** In anovulatory dysfunctional uterine bleeding, prolonged and unopposed estrogen stimulation causes progressive hyperplasia and proliferation of the uterine membrane, leading to advanced adenocystic or adenomatous hyperplasia, and even progression to endometrial carcinoma. Due to the lack of progesterone antagonism and glandular secretion, the uterine membrane thickens, glands proliferate, glandular cavities expand, and glandular epithelium undergoes abnormal hyperplasia. Blood supply to the membrane increases, with spiral arterioles becoming tortuous and coiled. The estrogen-induced polymerization and gelation of acidic mucopolysaccharides (AMPS) reduce vascular permeability in the stroma, impairing material exchange and causing local ischemia, necrosis, and shedding of the membrane, resulting in bleeding. Additionally, AMPS aggregation hinders the shedding of the uterine membrane, leading to asynchronous detachment and prolonged irregular bleeding.In ovulatory dysfunctional uterine bleeding, the corpus luteum may degenerate prematurely, shortening the luteal phase and causing frequent menstruation, or it may persist inadequately, with continued progesterone secretion leading to premenstrual bleeding, prolonged menstruation, or persistent spotting—or a combination of these. The mechanism involves insufficient secretion of estrogen and progesterone, particularly progesterone, preventing complete secretory transformation of the uterine membrane. Immature gland, stromal, and vascular development, along with asynchronous withdrawal of estrogen and progesterone, results in irregular shedding of the uterine membrane and abnormal bleeding.
TXA2, produced in platelets, induces vasoconstriction, platelet aggregation, thrombus formation, and hemostasis. In contrast, PGI2, synthesized in vascular walls, acts as a potent vasodilator, opposing TXA2 by inhibiting platelet aggregation and preventing thrombosis. Its activity is 20–30 times that of PGE1 and 10–15 times that of PGD2. PGI2 also suppresses platelet aggregation induced by arachidonic acid, ADP, and collagen, and reverses procoagulant effects of endogenous/exogenous factors. The functional coordination and dynamic balance between TXA2 and PGI2 are crucial for maintaining normal uterine membrane bleeding and hemostasis. Their effects are regulated by sex hormones, adrenergic nerve activity, and uterine muscle contractions.
The human uterus muscle and inner membrane contain two types of PG receptors (R1 and R2), which have strong affinity for PGE2 and PGF2α respectively, with PGA and E causing relaxation, while PGE2 and F2α constrict microvessels and microcirculation; whereas for the uterine myometrium, PGI2, E1, and D2 have a relaxing effect, while PGD2 and H2 have a constricting effect.
3. Abnormalities in the structure and function of the uterine endometrial spiral arterioles and lysosomes.
Abnormal spiral arterioles disrupt the microcirculatory function of the uterine endometrium, affecting the shedding of the functional layer and the repair of blood vessels and epithelium at the detachment surface. This impairs vasomotor function and local coagulation-fibrinolysis, leading to abnormal uterine bleeding.
Ultrastructural studies of the uterine endometrium confirm that the number and enzymatic activity of lysosomes progressively increase from the follicular phase to the luteal phase. Progesterone stabilizes while estrogen destabilizes lysosomal membranes. Thus, when progesterone levels drop before menstruation or when the estrogen/progesterone ratio is imbalanced (as in dysfunctional uterine bleeding), lysosomal membrane stability is compromised, leading to the release of phospholipase A2 into the cytoplasmic cells. This triggers arachidonic acid activation and a cascade of PGs formation. Additionally, lysosomal membrane rupture releases destructive hydrolases, causing endometrial cell rupture, layer collapse, necrosis, and bleeding.
4. Activation of the coagulation and fibrinolysis systems Observations indicate that dysfunctional uterine bleeding is often accompanied by deficiencies in coagulation factors V, VII, X, and XII, thrombocytopenia, anemia, iron deficiency, and Minot-Von Willebrand syndrome. Simultaneously, the endometrium shows increased levels and activity of plasminogen activators, which convert plasminogen to plasmin. Plasmin degrades fibrin, elevating fibrin degradation products (FDP) and reducing plasma fibrinogen, creating an afibrinogenemia state in the uterus. This disrupts normal coagulation and hemostasis at the tips of spiral arterioles and vascular lakes, resulting in prolonged and heavy bleeding.
bubble_chart Pathological Changes
I. Pathological Changes in the Uterine Endometrium of Anovulatory Dysfunctional Uterine Bleeding (DUB)
(1) Proliferative Endometrium: The most common type. The histological appearance is similar to that of the normal proliferative phase but persists into the premenstrual phase (Photo 1).
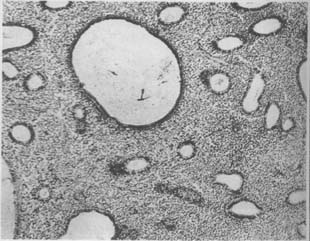
Photo 1: Hyperplasia of the Uterine Endometrium
(2) Cystic Hyperplasia of the Endometrium: Also known as Swiss cheese hyperplasia. The endometrium becomes thickened and polypoid, with an increased number of glands and dilated glandular cavities of irregular shapes, resembling Swiss cheese. The glandular epithelium is tall columnar and exhibits stratified or pseudostratified hyperplasia. The stroma shows edema, with underdeveloped spiral arterioles. The superficial microvessels of the endometrium are tortuous, congested, necrotic, or show focal hemorrhage.
(3) Adenomatous Hyperplasia of the Endometrium: The number of glands is significantly increased, varying in size and densely packed in a "back-to-back" arrangement. The glandular epithelium shows marked pseudostratified or papillary hyperplasia protruding into the glandular lumen. The nuclei are large, centrally located, deeply stained, with clear nuclear-cytoplasmic boundaries. Occasional mitotic figures may be seen (Photo 2).
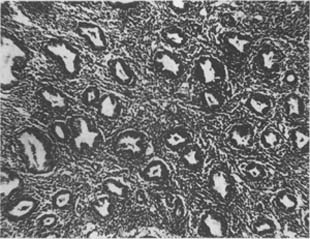
Photo 2: Adenomatous Hyperplasia of the Uterine Endometrium
(4) Atypical Hyperplasia of the Endometrium: On the basis of adenomatous hyperplasia, the glandular epithelium exhibits highly active mitotic figures, nuclear atypia, variation in nuclear size, deep staining, indistinct nuclear-cytoplasmic boundaries, and loss of polarity (Photo 3).
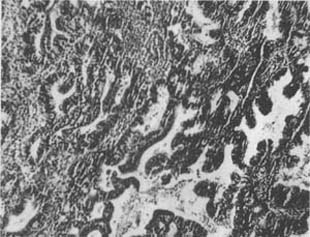
Photo 3: Atypical Hyperplasia of the Uterine Endometrium
Different types of hyperplastic endometrium account for over 90% of anovulatory DUB and 30.8–39.4% of all DUB cases (based on 31 studies analyzing 4,850 cases of DUB). It is noted that adenomatous and atypical hyperplasia are considered precancerous lesions of endometrial carcinoma, warranting close clinical attention and aggressive treatment.
II. Pathological Changes in the Uterine Endometrium of Ovulatory DUB
(1) Irregular Maturation Endometrium: Detection rate of 21%. Caused by inadequate luteal function and insufficient progesterone secretion, clinically presenting as a shortened luteal phase and frequent menstruation. Endometrial examination before menstruation reveals a mixture of secretory and incomplete secretory endometrium. The characteristic feature is normal secretory changes around blood vessels, while areas distant from vessels show incomplete secretory changes, with poorly developed glands (Grade I curvature), scant glandular secretion, and elongated oval nuclei. The stroma lacks a decidual reaction.
(2) Irregular Shedding Endometrium: Detection rate of 11%. Caused by incomplete regression of the corpus luteum and persistent but insufficient progesterone secretion, leading to prolonged or spotting menstruation. Endometrial examination after 5 days of bleeding shows a mixture of degenerated secretory endometrium and newly proliferative endometrium. The secretory glands appear in a plum-flower or star-like pattern. The glandular epithelium has abundant, clear cytoplasm and pyknotic nuclei, with dense stroma and degenerated spiral arterioles. Some areas may still show hemorrhage. This pattern is also seen in cases of uterine fibroids and endometrial polyps.
III. Atrophic Endometrium
Detection rate of 1.9–21.9%, most commonly seen in perimenopausal women with DUB.
The histopathological changes in the ovaries of DUB vary with age and type. In adolescent DUB, the ovaries are enlarged with retention follicular cysts (diameter ≥3 cm) and no corpus luteum formation; some cases show polycystic ovarian changes or luteinized unruptured follicles (LUFS).
In reproductive-age DUB, the ovaries are normal, but corpus luteum cysts may be observed. In perimenopausal DUB, the ovaries also exhibit polycystic changes, with the cortex filled with follicles or follicular cysts of varying sizes. Microscopically, stromal cell hyperplasia and hilus cell hyperplasia may be seen.
- Anovulatory dysfunctional uterine bleeding (DUB): Divided into two groups based on age.
- Adolescent DUB: Seen in post-menarche girls, caused by immaturity of the HPOU axis leading to failure to establish regular ovulation. Clinical manifestations include infrequent menstruation after menarche, followed by irregular hypermenorrhea, prolonged menstruation, and persistent spotting, resulting in severe anemia.
- Perimenopausal DUB: Refers to DUB in women aged ≥40 years around menopause, with the incidence of anovulatory DUB increasing yearly. Clinical manifestations include frequent menstruation, irregular cycles, excessive menstrual flow, and prolonged menstruation. In 10–15% of patients, severe irregular hypermenorrhea, menorrhagia and metrostaxis, and severe anemia occur. Endometrial biopsy often shows varying degrees of endometrial hyperplasia, so diagnostic curettage is necessary, with particular attention to excluding non-DUB uterine bleeding caused by gynecological tumors (uterine fibroids, endometrial cancer, ovarian cancer, cervical cancer).
- Ovulatory DUB: Most common in women of reproductive age, but also seen in some adolescent girls and perimenopausal women. Clinically classified into the following types:
- Ovulatory menstrual disorders
- Ovulatory infrequent menstruation: Seen in adolescent girls. After menarche, the follicular phase is prolonged, while the luteal phase remains normal, with cycles ≥40 days, infrequent menstruation, and hypomenorrhea, often a precursor to polycystic ovaries. Rarely seen in perimenopausal women nearing menopause, it often progresses to natural menopause.
- Ovulatory frequent menstruation: In adolescent girls, increased ovarian sensitivity to gonadotropins accelerates follicular development, shortening the follicular phase and leading to frequent menstruation, while ovulation and the luteal phase remain normal. In perimenopausal women, both the follicular and luteal phases shorten, leading to early menopause.
- Luteal phase dysfunction
- Luteal phase defect (LPD): Early degeneration of the corpus luteum, shortening the luteal phase to ≤10 days. Clinical manifestations include frequent menstruation, shortened cycles, premenstrual bleeding, and hypermenorrhea, often accompanied by infertility and early or late abortion. Endometrial pathology shows irregular ripening or incomplete secretion.
- Incomplete luteal regression: Also called prolonged luteal function, where the corpus luteum fails to fully regress within 3–5 days, or regression is delayed, or it continues to secrete progesterone during menstruation, leading to irregular shedding of the endometrium. Prolonged menstruation and persistent spotting occur; if combined with early luteal regression, frequent menstruation and hypermenorrhea may manifest. Common after artificial late abortion or induced labor, and often associated with uterine fibroids, endometrial polyps, and adenomyosis.
- Ovulatory menstrual disorders
- Midcycle [second phase] bleeding: Also called ovulation bleeding. Often accompanied by ovulation pain (intermenstrual pain or mittelschmerz), caused by ovulation stimulation and estrogen fluctuations, resulting in slight bleeding (1–3 days) and abdominal pain. In some cases, heavier bleeding persists into the menstrual phase, mimicking frequent menstruation (pseudopolymenorrhea).
bubble_chart Clinical Manifestations
Characterized by menstrual cycle disorders and changes in the quantity and nature of uterine bleeding, it can be divided into the following types:
- Oligomenorrhea: Irregular uterine bleeding with cycles ≥40 days, often accompanied by hypomenorrhea.
- Polymenorrhea: Irregular uterine bleeding with cycles ≤21 days, often accompanied by hypermenorrhea.
- Hypermenorrhea (hypermenorrhea or menorrhagia): Refers to excessive menstrual flow and/or prolonged menstruation with regular cyclic uterine bleeding.
- Metrorrhagia: Refers to irregular menstrual cycles without excessive flow.
- Menometrorrhagia: Refers to irregular menstrual cycles accompanied by excessive flow and prolonged menstruation.
- Hypomenorrhea: Refers to regular menstrual cycles with only reduced flow.
- Intermenstrual bleeding [second stage]: Refers to slight uterine bleeding between two normal regular menstruations, often accompanied by ovulation and ovulation pain.
The aim is to determine the disease cause, pathology, and clinical classification of abnormal uterine bleeding, and to exclude bleeding caused by organic sexually transmitted diseases of the reproductive tract.
- Medical history: Carefully inquire about personal developmental history and menstruation history (age of menarche, cycle, duration, flow, accompanying symptoms and signs), disease cause and predisposing factors, onset conditions, and diagnostic and treatment process. Special attention should be paid to the names, doses, efficacy of hormones and medications used, as well as the pathological results of hormone assays and endometrial curettage.
- Physical examination: Pay attention to overall nutritional status, presence of anemia, hematological diseases, symptoms and signs of bleeding disorders (petechiae, ecchymosis, purpura, and jaundice), lymph node, thyroid, and breast examinations. Check for abdominal or pelvic masses and hepatosplenomegaly.
- Gynecological examination: For unmarried women, only perform abdominal palpation. Married women should undergo routine bimanual and rectovaginal examinations. Observe the amount, source, and nature of bleeding, and check the cervix, uterus, and ovaries for tumors, inflammation, endometriosis, or other organic lesions. Rectal examination helps assess the posterior pelvis and rectum.
- Auxiliary examinations: Aim to evaluate ovarian function (ovulation and luteal function) and the histopathological changes of the endometrium.
- Diagnostic curettage: To monitor ovulation, perform curettage 1–2 days before menstruation or within the first 6 hours of menstruation. To determine the type of dysfunctional uterine bleeding, perform curettage after the fifth day of menstruation. Curettage serves both diagnostic and therapeutic purposes, so it must be thorough and comprehensive, especially focusing on the bilateral cornual regions. All curettage specimens should be sent for examination. Except for unmarried adolescents, diagnostic curettage is an essential step in the management of dysfunctional uterine bleeding.
- Ovulation and luteal function monitoring:
- Basal body temperature (BBT): A biphasic curve suggests ovulation, while a shortened (<8 days) or unstable high-temperature phase indicates luteal phase dysfunction. A monophasic curve suggests anovulation.
- Vaginal cytology and cervical mucus function (quantity, viscosity, spinnbarkeit, and crystallization pattern) examination: Assess ovulation and luteal function.
- Hormone assays: Include FSH, LH, PRL, E2, P, TO, 17KS, 17OHCS, T3, T4, etc.
- Ultrasound examination: Observe follicular development, ovulation, and luteal status, and exclude ovarian tumors.
- Blood and coagulation/fibrinolysis function tests: Include hemoglobin, red blood cells, white blood cells, hematocrit, bleeding and clotting time, prothrombin time, serum iron measurement, and bone marrow puncture if necessary.
- Liver and kidney function tests: Include total protein, A/G ratio, transaminases (GOT, GPT, γ-GT), bilirubin, BUN, blood glucose, and lipid profile.
bubble_chart Treatment Measures
The treatment principles, methods, medications, and monitoring are determined based on the patient's age, type of dysfunctional uterine bleeding (DUB), endometrial pathology, and fertility requirements. Systematic treatment of DUB includes: eliminating the disease cause, rapidly stopping bleeding, regulating menstruation, restoring function, and preventing recurrence.
I. Treatment of Anovulatory DUB For adolescent anovulatory DUB, the treatment principles are to promote ovulation, establish regular menstruation, and prevent recurrence. For perimenopausal anovulatory DUB, the focus is on curbing endometrial hyperplasia, inducing menopause, and preventing carcinogenesis.
(1) Hemostasis: Methods include curettage, hormonal therapy, and drug therapy.
1. Curettage: Except for unmarried women, curettage can rapidly and effectively stop bleeding in both ovulatory and anovulatory DUB, serving both diagnostic and therapeutic purposes. The curettage should be thorough, and all tissue removed should be sent for pathological examination. Menstrual regulation therapy should begin on the fifth day after the procedure based on endometrial pathology.
2. Sex Hormones: Include estrogen, progesterone, and androgen for hemostasis.
(1) High-dose estrogen hemostasis: Only used for adolescent DUB with mild anemia (Hb ≥ 80g/L). The principle is that high-dose estrogen rapidly promotes endometrial hyperplasia, repairing the wound to stop bleeding. The drawbacks include high dosage, severe gastrointestinal reactions, heavy withdrawal bleeding after discontinuation, and potential suppression of the hypothalamic-pituitary axis, so it is now less commonly used.
Method: Diethylstilbestrol or estradiol benzoate 2mg intramuscular injection every 6–8 hours. After 3–4 injections (24–36 hours) and cessation of bleeding, reduce the dose by one-third every 3 days to a maintenance dose of 1mg/d (intramuscular or oral). Discontinue 20 days after hemostasis. Menstrual regulation therapy begins on the fifth day of withdrawal bleeding.
(2) High-dose progesterone hemostasis: Suitable for all age groups and types of DUB. The principle is to promote synchronous secretory transformation of the endometrium to stop bleeding, followed by concentrated withdrawal bleeding after discontinuation.
Method: ① Oral: Norethisterone 5.0–7.5mg, or medroxyprogesterone acetate (MPA) 8–10mg, every 4–6 hours. After 3–4 doses (24–36 hours) and cessation of bleeding, reduce to every 8 hours. Then decrease the dose by one-third every 3 days to a maintenance dose (norethisterone 2.5–5.0mg/d or MPA 4–6mg/d). Discontinue 20 days after hemostasis. To prevent breakthrough bleeding, a small dose of estrogen (e.g., diethylstilbestrol 0.25–0.5mg/d) can be added in the evening. Menstrual regulation therapy begins on the fifth day of withdrawal bleeding. ② Intramuscular: Hydroxyprogesterone caproate compound (250mg hydroxyprogesterone caproate + 5mg estradiol valerate per ampoule) 1 ampoule intramuscularly, achieving hemostasis in 1–2 days. Repeat the injection on days 7–10 to complete one treatment cycle. To accelerate hemostasis, estradiol benzoate 2mg or compound progesterone (20mg progesterone + 2mg estradiol benzoate per ampoule) can be added. After hemostasis, inject one ampoule of compound progesterone weekly for 3–4 doses to complete one cycle. Menstrual regulation therapy begins on the fifth day of withdrawal bleeding. ③ Medical curettage: Suitable for minor, prolonged bleeding without recent heavy bleeding. The principle is to use progesterone to induce secretory transformation of the endometrium followed by concentrated withdrawal. Method: Progesterone 20mg/d × 3–5 days, with concentrated withdrawal bleeding occurring 3–5 days after discontinuation and stopping naturally. To reduce bleeding, testosterone propionate 25–50mg/d can be added. Alternatively, one ampoule of trihormone (a combination of progesterone, estradiol, and testosterone) per day × 3 days can be used for medical curettage. Menstrual regulation therapy begins on the fifth day of withdrawal bleeding.
(3) Androgens: Used only as adjuvant therapy to estrogen and progestogen for hemostasis, aiming to counteract estrogen, reduce pelvic congestion, enhance uterine muscle tone, and decrease bleeding volume. However, they cannot shorten bleeding duration or achieve complete hemostasis. Use with caution in adolescent girls. Testosterone propionate 25–50 mg/day for 3–5 days, then reduced to 1–2 times per week, with a total cycle dose not exceeding 300 mg.
3. Drug therapy: Includes comprehensive measures such as hemostatic medicinals, antifibrinolytic drugs, prostaglandin synthetase inhibitors, clotting factors, uterine contractions, and blood transfusion.
(1) Hemostatic medicinals: Aim to improve platelet function, shorten clotting time, reduce vascular fragility and permeability, improve microcirculation, and stimulate hematopoiesis. Methods: Dicynone 250–500 mg intramuscular injection or intravenous drip; Adrenosin 5–10 mg intramuscular injection; oral administration of vitamin K and C.
(2) Antifibrinolytic drugs: Aim to inhibit fibrinolysis and suppress plasminogen activator. Methods: ① EACA 4–6 g added to 10% glucose solution 100 ml for rapid drip (15–30 minutes), then adjusted to a maintenance rate of 1 g/h, with a total daily dose of 6–12 g; ② PAMBA 300–500 mg added to 10% glucose solution 100–200 ml for drip, with a total daily dose of 600–1000 mg; ③ Trans-AMCA 200–300 mg added to 10% glucose solution for drip, with a total daily dose of 400–600 mg.
(3) Prostaglandin synthetase inhibitors: ① Indomethacin 25 mg × 3/day; ② Mefenamic acid 250 mg × 3/day; ③ Chlofenamic acid 200 mg × 3/day.
(4) Clotting factors and blood transfusion: Such as fibrinogen, platelet infusion, and fresh blood transfusion. Chinese medicinals such as Sanqi and Yunnan Baiyao also have good hemostatic effects. Uterine contractions have no significant hemostatic effect.
(II) Cycle regulation: Based on hemostatic treatment, this method mimics the rhythm of reproductive hormones using estrogen-progestin artificial cycle therapy to promote the cyclical development and shedding of the uterine membrane, improve HPO axis feedback function, and may result in rebound ovulation and restoration of regular menstruation after discontinuation.
1. Full-cycle therapy
(1) Sequential estrogen-progestin therapy: Suitable for adolescent dysfunctional uterine bleeding. Starting from the fifth day of the menstrual cycle, oral administration of diethylstilbestrol 0.5–1.0 mg/day × 20–22 days. For the last 10 days, add medroxyprogesterone 8–10 mg/day, or for the last 5 days, inject progesterone 20 mg/day. One course consists of 3 cycles.
(2) Combined estrogen-progestin therapy: Suitable for reproductive-age and perimenopausal dysfunctional uterine bleeding, endometrial hyperplasia, and hypermenorrhea. ① Oral contraceptive I or II (full or half dose) starting from the fifth day of the menstrual cycle, 1 tablet/day × 22 days, for 3 cycles. ② Medroxyprogesterone 4 mg + diethylstilbestrol 0.5 mg/day, or norethisterone 2.5 mg + diethylstilbestrol 0.5 mg/day × 20–22 days, for 3 cycles.
(3) Progestin therapy: Norethisterone 2.5–5.0 mg/day; or megestrol, medroxyprogesterone 4–8 mg/day; or chlormadinone 12 mg/day × 20–22 days. One course consists of 3 cycles.
(4) Progestin-androgen therapy: Based on progestin therapy, add methyltestosterone 5–10 mg sublingual daily to enhance suppression of the HPOU axis.
2. Luteal phase therapy: Limited to cycle regulation, supporting the corpus luteum, and controlling bleeding. Methods: From the 15th–24th day of the menstrual cycle (luteal phase), administer estrogen-progestin orally or via intramuscular injection for 10 days. Drugs include: ① Oral contraceptive I or II (full or half dose)/day; ② Norethisterone 2.5–5.0 mg, or megestrol, medroxyprogesterone 6–8 mg + diethylstilbestrol 0.25–0.5 mg/day; ③ Compound progesterone injection 1 ampoule/day × 5–7 days (days 21–25 of the cycle).
(3) Ovulation induction therapy: Suitable for adolescent anovulatory dysfunctional uterine bleeding and women of childbearing age with dysfunctional uterine bleeding who desire pregnancy. Ovulation induction therapy can fundamentally prevent the recurrence of dysfunctional uterine bleeding.
Ovulation induction therapy is guided by reproductive hormone measurements, with appropriate selection of ovulation-inducing drugs and combinations: ①CC-hCG; ②hMG-hCG; ③GnRHa pulse therapy; ④Bromocriptine therapy, etc.
(4) Suppress endometrial hyperplasia, prevent carcinogenesis, and induce menopause. Suitable for perimenopausal anovulatory dysfunctional uterine bleeding (DUB) with endometrial hyperplasia (cystic glandular/adenomatous type) or combined with uterine fibroids or endometriosis. Commonly used drugs and therapies include:
1. Danazol 200mg×3/day, orally.
2. Nemestran (R2323, Gestrinone) 2.5mg×2/week, orally.
3. Tamoxifen 20–40mg/day, orally.
4. GnRHa 300–500μg/day, 1H.
The above drugs are administered in 3-month courses. Repeat treatment if necessary.
(5) Surgical therapy: Suitable for cases unresponsive to or recurrent after hormonal or drug therapy. Methods include: microwave, infrared, liquid nitrogen cryotherapy, laser, or microsurgical endometrial ablation via hysteroscopy. For perimenopausal women with adenomatous or atypical endometrial hyperplasia, combined with uterine fibroids, adenomyosis, or severe anemia, hysterectomy may be performed.
II. Treatment of ovulatory DUB The principle is to suppress hypermenorrhea, support luteal function, regulate the cycle, and prevent recurrence.
(1) Suppress hypermenorrhea: ① Combined estrogen-progestogen therapy throughout the cycle; ② Progestogen cyclic therapy; ③ Progestogen-androgen therapy; ④ Androgen therapy: Starting from the fifth day of the menstrual cycle, oral methyltestosterone 10mg/day×20–22 days or testosterone propionate 25mg×2/week for 4 weeks; ⑤ Combined estrogen-progestogen therapy in the latter half of the cycle; ⑥ Prostaglandin synthase inhibitors; ⑦ Anti-estrogen-progestogen therapy (Danazol, Nemestran, Tamoxifen, etc.).
(2) Support luteal function
1. Ovulation induction therapy: Suitable for poor follicular maturation, luteal insufficiency, infertility, and habitual late abortion. Methods: ① CC-hCG; ② hMG-hCG; ③ pFSH-hCG; ④ GnRHa therapy, etc.
2. Support luteal function: Suitable for luteal insufficiency or incomplete regression. Methods: ① hCG therapy: Intramuscular injection of hCG 5000–10000 IU at ovulation, followed by 5000 IU 5 days later to support the corpus luteum. Alternatively, 2000 IU/day on days 4, 6, and 8 post-ovulation; ② CC therapy; ③ Progesterone therapy: Oral medroxyprogesterone 4–8mg/day×10 days post-ovulation, or intramuscular progesterone 10–20mg/day×5–7 days starting 7 days after BBT rise; ④ Combined estrogen-progestogen therapy in the latter half of the cycle; ⑤ Bromocriptine therapy: For cases with hyperprolactinemia, oral bromocriptine 2.5mg/day starting from the fifth day of the menstrual cycle; ⑥ Dexamethasone therapy: For cases with hyperandrogenemia, 0.5mg/day.
III. Treatment of complications DUB is often complicated by anemia, hypoproteinemia, and malnutrition, so supportive therapy should be strengthened. Additionally, DUB may be the initial symptom of systemic diseases (e.g., aplastic anemia, leukemia, thrombocytopenic purpura, hypersplenism, cirrhosis) or coexist with endocrine-metabolic disorders (thyroid, adrenal diseases, diabetes) and gynecological conditions (uterine fibroids, endometrial polyps, pelvic congestion syndrome, polycystic ovaries, ovarian functional tumors, endometrial cancer). Therefore, active treatment of the primary disease and comorbidities is crucial.
The purpose is to exclude abnormal uterine bleeding caused by organic sexually transmitted diseases. The causes of abnormal uterine bleeding in women of different ages are:
- Neonatal and childhood period
- Maternal estrogen influence
- Grape-like fleshy tumor
- Ovarian cancer
- Injury
- Infection
- Foreign body
-
Adolescence
- Psychological trauma, stress
- Immaturity of the hypothalamic-pituitary-ovarian axis
- Luteal phase dysfunction
- Malnutrition
-
Reproductive age
- Pregnancy complications
- Ectopic pregnancy
- Retained placenta, placental polyp
- Late abortion
- Trophoblastic diseases (hydatidiform mole, invasive mole, choriocarcinoma)
-
Anovulatory type
- Central: Nervous system tumors, psychological trauma
- Endocrine: Thyroid disorders, adrenal diseases, metabolic diseases
- Gonadal: Polycystic ovary syndrome
- Target organ: Endometrial hyperplasia
- Organic sexually transmitted diseases: Ovarian functional tumors
-
Ovulatory type
- Frequent menstruation (shortened follicular or luteal phase)
- Irregular shedding of the endometrium
- Blood, coagulation, and fibrinolysis mechanism abnormalities
- Persistent corpus luteum syndrome (Halban's syndrome)
- Iatrogenic factors (anticoagulant drugs, IUD)
- Organic sexually transmitted diseases (tumors, inflammation, submucous fibroids)
- Pregnancy complications
-
Perimenopausal period
- Carcinoma of endometrium
- Cervical cancer
- Cervical polyp
-
Late postmenopausal stage [third stage]
- Exogenous estrogen
- Cervical cancer
- Carcinoma of endometrium
- Ovarian cancer
- Atrophic vaginitis






