| disease | Pleural Effusion |
| alias | Pleural Fluid |
Normally, there are 3–15 ml of fluid in the pleural cavity, which serves as a lubricant during respiration, but the volume of fluid in the pleural cavity is not constant. Even in healthy individuals, 500–1000 ml of fluid is formed and reabsorbed every 24 hours. The fluid in the pleural cavity is reabsorbed through the venous end of the capillaries, while the remaining fluid is returned to the bloodstream via the lymphatic system, maintaining a dynamic balance between filtration and absorption. If systemic or local disorders disrupt this dynamic equilibrium, causing excessive fluid formation or delayed absorption in the pleural cavity, it clinically results in pleural effusion.
bubble_chart Etiology
Laboratory examination: {|###|}I. Increased hydrostatic pressure in pleural capillaries {|###|}Such as congestive heart failure, constrictive pericarditis, increased blood volume, obstruction of the superior vena cava or azygos vein, resulting in pleural transudate. {|###|}II. Increased permeability of pleural capillaries {|###|}Such as pleural inflammation (tuberculosis, pneumonia), connective tissue diseases (systemic lupus erythematosus, rheumatoid arthritis), pleural tumors (malignant metastasis, mesothelioma), pulmonary infarction, subdiaphragmatic inflammation (subphrenic abscess, liver abscess, acute pancreatitis), etc., resulting in pleural exudate. {|###|}III. Decreased colloid osmotic pressure in pleural capillaries {|###|}Such as hypoalbuminemia, liver cirrhosis, nephrotic syndrome, acute glomerulonephritis, myxedema, etc., resulting in pleural transudate. {|###|}IV. Impaired lymphatic drainage of the parietal pleura {|###|}Such as lymphatic obstruction by cancer, developmental lymphatic drainage abnormalities, etc., resulting in pleural exudate. {|###|}V. Hemorrhage in the pleural cavity due to injury {|###|}Such as rupture of aortic aneurysm, esophageal rupture, thoracic duct rupture, etc., resulting in hemothorax, pyothorax, or chylothorax. {|###|}The main etiologies of pleural effusion and the nature of the effusion are shown in Table 1. {|###|}Table 1: Main etiologies and nature of pleural effusion {|###|} {|###|} {|###|} {|###|} {|###|} {|###|} {|###|}Transudate {|###|} {|###|}Exudate (serous or bloody) {|###|} {|###|}Pyothorax {|###|} {|###|}Hemothorax {|###|} {|###|}Chylothorax {|###|} {|###|} {|###|} {|###|}Infectious diseases {|###|} {|###|} {|###|} {|###|}Pleuritis (tuberculosis, various infections), subdiaphragmatic inflammation {|###|} {|###|}Pulmonary tuberculosis, various pulmonary infections {|###|} {|###|}Pulmonary tuberculosis {|###|} {|###|} {|###|} {|###|} {|###|} {|###|}Tumors, circulatory system disorders {|###|} {|###|}Obstruction of superior vena cava, congestive heart failure, constrictive pericarditis {|###|} {|###|}Malignant tumors, pleural mesothelioma, pulmonary infarction {|###|} {|###|} {|###|} {|###|}Malignant tumors, rupture of hemangioma, pulmonary infarction {|###|} {|###|}Obstruction of thoracic duct {|###|} {|###|} {|###|} {|###|}Hypoalbuminemia {|###|} {|###|}Nephrotic syndrome, liver cirrhosis {|###|} {|###|} {|###|} {|###|} {|###|} {|###|} {|###|} {|###|} {|###|}Other disorders {|###|} {|###|}Peritoneal dialysis, myxedema, drug allergy, radiation reaction {|###|} {|###|}Rheumatic fever, systemic lupus erythematosus, post-thoracic surgery, pneumothorax {|###|} {|###|}Trauma, esophageal fistula, pneumothorax, secondary pyogenic infection after thoracentesis {|###|} {|###|}Trauma, pneumothorax (with tearing of pleural adhesions) {|###|} {|###|}Trauma-induced thoracic duct rupture, filariasis {|###|} {|###|} {|###|} {|###|} {|###|}
Pleural effusion is most commonly caused by exudative pleuritis; subcutaneous nodule disease is particularly common among young and middle-aged patients. In middle-aged and elderly patients with pleural effusion (especially bloody pleural fluid), careful consideration should be given to malignant sexually transmitted diseases and malignant tumors (such as lung cancer, breast cancer, lymphoma, etc.) metastasizing to the pleura or mediastinal lymph nodes, which can cause pleural effusion. Tumor involvement of the pleura, increasing its surface permeability, or obstruction of lymphatic drainage, or accompanying obstructive pneumonia affecting the pleura, can all lead to exudative pleural effusion. Occasionally, obstruction of the thoracic duct can result in chylothorax. When the pericardium is affected, pericardial effusion may occur, or obstruction of the superior vena cava may increase intravascular hydrostatic pressure, or malnutrition and hypoalbuminemia caused by malignant tumors can lead to fistula disease effusion.The mechanism of pleural effusion and absorption
In healthy individuals, the pleural cavity maintains a negative pressure (averaging -5cmH2O during respiration, where 1cmH2O = 98Pa). The pleural fluid contains proteins, contributing to its colloid osmotic pressure (8cmH2O). The formation and resolution of pleural fluid are closely related to the osmotic and hydrostatic pressures in the pleural capillaries. The parietal pleura is supplied by the systemic circulation, with high capillary hydrostatic pressure (30cmH2O), while the visceral pleura is supplied by the pulmonary circulation, with lower venous pressure (11cmH2
O). The systemic and pulmonary circulations absorb blood at equal rates (Figure 1).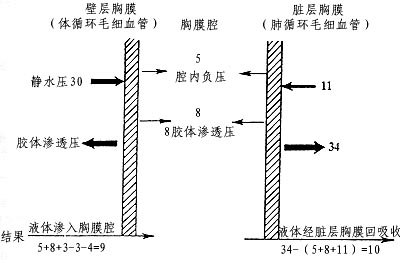
Figure 1 Schematic diagram of pleural fluid circulation and related pressures (cmH2O)
Based on animal experiments, it is estimated that 0.5 to 1 liter of fluid can pass through the human pleural cavity daily. Proteins in the pleural fluid primarily enter the thoracic duct via lymphatic vessels.
Pleural inflammation can increase vascular permeability, allowing more proteins to enter the pleural cavity and raising the osmotic pressure of the pleural fluid. Tumors may compress or obstruct lymphatic drainage, leading to protein accumulation in the pleural fluid and resulting in pleural effusion. Portal hypertension cirrhosis often causes hypoalbuminemia, reducing plasma colloid osmotic pressure and potentially producing transudative fluid. When ascites is present, pleural effusion may also occur through congenital diaphragmatic defects or lymphatic pathways. Allergic diseases, autoimmune disorders, cardiovascular diseases, or thoracic trauma can also contribute to the development of pleural effusion.
bubble_chart Clinical Manifestations
Age, medical history, symptoms, and signs all have diagnostic value. Subcutaneous nodular pleuritis is more common in young people and often presents with fever; patients over middle age should be alert to pleural metastasis caused by lung cancer. Inflammatory effusions are mostly exudative and often accompanied by chest pain and fever. Pleural effusion caused by heart failure is a transudate. Right-sided pleural effusion associated with liver abscess may be reactive pleuritis or empyema. Symptoms are often not obvious when the effusion volume is less than 0.3L; if it exceeds 0.5L, the patient may gradually feel chest tightness. Local dullness on percussion and reduced breath sounds may be present. As the effusion volume increases, the two layers of pleura separate and no longer rub during respiration, so chest pain gradually eases, but dyspnea worsens. With a large effusion, mediastinal organs are compressed, leading to more pronounced palpitations and dyspnea.
1. Appearance
The fluid from fistula disease is transparent and clear, does not coagulate upon standing, and has a specific gravity <1.016~1.018。滲出液則多呈草黃色稍混濁,比重>1.018. Purulent pleural effusion caused by large intestine bacilli or anaerobic infections often has a foul odor. Bloody pleural effusion may appear as varying degrees of meat-washing water or venous blood; milky pleural effusion indicates chylothorax; if the pleural fluid is chocolate-colored, consider the possibility of an amoebic liver abscess rupturing into the pleural cavity; black pleural fluid may suggest aspergillus infection.
2. Cells
Normal pleural fluid contains a small number of mesothelial cells or lymphocytes. During pleural membrane inflammation, various inflammatory cells, as well as proliferating and degenerating mesothelial cells, may be observed in the pleural fluid. The cell count in fistula disease effusion is usually less than 100×106/L, primarily consisting of lymphocytes and mesothelial cells. Exudative effusion often has a white blood cell count exceeding 500×106/L. In empyema, the white blood cell count can reach over 1000×106/L. An increase in neutrophils suggests acute inflammation; a predominance of lymphocytes often indicates subcutaneous node-related or malignant conditions. In cases of Chinese Taxillus Herb infection or connective tissue disease, eosinophils are often elevated. When red blood cells in the pleural fluid exceed 5×109/L, the fluid may appear light red, often caused by malignancy or subcutaneous node involvement. Pleural puncture injury to blood vessels can also lead to bloody pleural effusion, requiring careful differentiation. If red blood cells exceed 100×109/L, consider trauma, tumors, or pulmonary infarction. Malignant cells can be detected in approximately 60% of malignant pleural effusions, and repeated examinations can improve detection rates. Malignant tumor cells in pleural fluid often exhibit characteristics such as enlarged and irregular nuclei, nuclear aberrations, hyperchromasia, abnormal nuclear-to-cytoplasmic ratios, and abnormal mitotic figures, which should be carefully differentiated. Mesothelial cells in pleural fluid often appear deformed and can be misdiagnosed as tumor cells. In non-subcutaneous node-related pleural effusion, intermediate cells exceed 5%, while in subcutaneous node-related effusion, they are usually below 1%. In systemic lupus erythematosus with pleural effusion, the antinuclear antibody titer in the pleural fluid may reach 1:160 or higher, and lupus cells are easily identified.
3. pH
The pH of subcutaneous node-related pleural fluid is often <7.30;pH<7.00者僅見於膿胸以及食管破裂所致胸腔積液。急性胰腺炎所致胸液的pH<7.30;若pH<7.40,應考慮惡性胸液。
4. Pathogens
Microscopic examination and culture of pleural fluid for bacteria aid in pathogen diagnosis. In subcutaneous node-related pleural membrane inflammation, culturing the sediment of pleural fluid for subcutaneous node bacteria yields a positive rate of only 20%. Chocolate-colored pus should be examined microscopically for amoebic trophozoites.
5. Proteins
The protein content in exudative effusion has a pleural fluid/serum ratio greater than 0.5. When the protein content is 30g/L, the specific gravity of the pleural fluid is approximately 1.018 (for every 1g change in protein, the specific gravity changes by 0.003). The protein content in fistula disease effusion is relatively low (<30g/L),以白蛋白為主,粘蛋白試驗(Rivalta試驗)陰性。
Carcinoembryonic antigen (CEA): Elevated CEA levels in malignant pleural effusion appear earlier and more significantly than in serum. If the pleural fluid CEA value exceeds 15–15μg/L or the pleural fluid/serum CEA ratio is >1, it often indicates malignant pleural effusion. Increased ferritin levels in malignant pleural effusion can serve as a reference for differential diagnosis. Combined testing of multiple markers can improve the positive detection rate.
6. Lipids
In chylothorax, the pleural fluid contains high levels of neutral fats and triglycerides (>4.52mmol/L), appearing as a milky turbidity, stained red by Sudan III, but with low cholesterol content, which can be seen in cases of thoracic duct rupture. "Chylous-like" or cholesterol pleural effusion (cholesterol >2.59mmol/L) is associated with chronic effusion cholesterol abdominal mass and can be observed in chronic subcutaneous node pleuritis, malignant pleural effusion, cirrhosis, or wind-dampness arthritis. Although cholesterol pleural effusion contains high levels of cholesterol, the triglyceride levels remain normal, appearing as light yellow or dark brown, containing cholesterol crystals, fat particles, and a large number of degenerated cells (lymphocytes, red blood cells).
7. Glucose
The glucose content in pleural fluid of normal individuals is similar to that in blood and changes with fluctuations in blood glucose levels. Measuring pleural fluid glucose levels helps differentiate the disease cause of pleural effusion. The glucose content in transudates and most exudates is normal; whereas in tuberculous, malignant, rheumatoid arthritis-related, and purulent pleural effusions, the glucose content may<3.35mmol/L。若胸膜病變範圍較廣,使葡萄糖及酸性代謝物難以透過胸膜,可使葡萄糖含量較低,提示腫瘤廣泛浸潤,其胸液中惡性腫瘤細胞發現率亦高。
8. Enzymes
An increase in pleural fluid lactate dehydrogenase (LDH) content, exceeding 200U/L, with a pleural fluid LDH/serum LDH ratio greater than 0.6, suggests an exudate. The LDH activity in pleural fluid reflects the degree of pleural membrane inflammation—the higher the value, the more pronounced the inflammation. LDH > 500U/L often indicates malignancy or bacterial infection complicating the pleural effusion.
Elevated pleural fluid amylase can be seen in acute pancreatitis, malignancies, etc. In acute pancreatitis accompanied by pleural effusion, amylase leakage due to fistula disease causes the enzyme level in pleural fluid to exceed that in serum. Some patients experience severe chest pain and dyspnea, which may mask abdominal symptoms. In such cases, pleural fluid amylase is already elevated, and clinical diagnosis should take note of this.
Adenosine deaminase (ADA) is present in higher concentrations in lymphocytes. In tuberculous pleuritis, due to stimulated cellular immunity, lymphocytes significantly increase, leading to pleural fluid ADA levels exceeding 100U/L (normally not exceeding 45U/L). Its diagnostic sensitivity for tuberculous pleuritis is relatively high.
9. Immunological Tests
With advances in cell biology and molecular biology, immunological testing of pleural fluid has gained attention. It plays a role in differentiating benign from malignant pleural effusions, studying the pathogenesis of pleural effusion, and potentially guiding future biological treatments for pleural effusion.
In tuberculous and malignant pleural effusions, T lymphocytes increase, particularly in tuberculous pleuritis, where they can reach up to 90%, predominantly T4(CD+4). In malignant pleural effusions, T-cell function is suppressed, and their cytotoxic activity against autologous tumor cells is significantly lower than that of peripheral blood lymphocytes, indicating localized immunosuppression in the pleural cavity of these patients. In pleural effusions caused by systemic lupus erythematosus and rheumatoid arthritis, complement components C3 and C4 are reduced, while immune complex levels are elevated.
10. Pleural Biopsy
Percutaneous pleural biopsy is helpful in determining the presence of tumors and diagnosing granulomatous sexually transmitted diseases of the pleura. When tuberculous disease is suspected, biopsy specimens can undergo pathological examination and tuberculous culture. Pleural biopsy is contraindicated in empyema or patients with bleeding tendencies. If necessary, biopsy can be performed via thoracoscopy.
Ultrasound Examination
Can differentiate pleural effusion, pleural thickening, and hydropneumothorax. It provides accurate localization for encapsulated effusions, aiding in thoracentesis.
Imaging Diagnosis
When the pleural effusion volume is 0.3–0.5L, chest X-ray only shows blunting of the costophrenic angle; larger effusions display a shadow with an outward and upward curved upper border (Figure 1). In the supine position, the effusion spreads out, reducing the radiolucency of the entire lung field. In hydropneumothorax, a fluid level is visible. Massive effusion causes complete opacification of the affected side, with mediastinal shift to the contralateral side. Effusions often have smooth, rounded margins and may be localized within the interlobar fissures or between the lung and diaphragm; ultrasound aids in diagnosis.
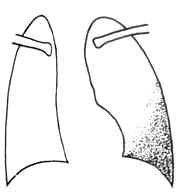
Figure 1 Exudative pleuritis
B-ultrasound can detect masses obscured by pleural fluid and assist in locating thoracentesis sites. CT scans can differentiate between exudates, blood, or pus based on the density of the pleural fluid and can also reveal mediastinal, paratracheal lymph nodes, pulmonary masses, pleural mesothelioma, and intrathoracic metastatic tumors. CT has high sensitivity and density resolution for pleural lesions, making it easier to detect small effusions that are difficult to visualize on plain X-rays.
bubble_chart Treatment Measures
Pleural effusion is part of systemic diseases of the chest, and treating the {|###|}disease cause{|###|} is particularly important. Effusion from {|###|}fistula disease{|###|} can often be absorbed after correcting the {|###|}disease cause{|###|}. Common {|###|}disease causes{|###|} of exudative pleuritis include {|###|}subcutaneous node{|###|} disease, malignant tumors, and pneumonia.
1. {|###|}Subcutaneous node{|###|} pleuritis
Most patients respond well to anti-{|###|}subcutaneous node{|###|} drug therapy. Small amounts of pleural effusion generally do not require drainage or may only need diagnostic puncture. Thoracentesis not only aids in diagnosis but also relieves pressure on the lungs, heart, and blood vessels, improves breathing, prevents fibrin deposition and pleural thickening, and avoids {|###|}injury{|###|} to lung function. After drainage, toxic symptoms may lessen, and {|###|}warm purgation{|###|} may decrease, helping the compressed lung to re-expand quickly. For large effusions, drainage should be performed 2–3 times per week until complete absorption. Each drainage should not exceed 1000 ml, as rapid or excessive drainage can cause a sudden drop in pleural pressure, leading to pulmonary {|###|}edema{|###|} or circulatory disturbances. This type of re-expansion pulmonary {|###|}edema{|###|} after rapid drainage manifests as severe coughing, shortness of breath, copious frothy sputum, diffuse moist rales in both lungs, and a drop in PaO2. Immediate oxygen therapy, glucocorticoids, diuretics, fluid restriction, and close monitoring of acid-base balance are required. If symptoms such as dizziness, {|###|}cold sweating{|###|}, {|###|}palpitation{|###|}, pale {|###|}complexion{|###|}, weak pulse, or cold limbs (a "pleural reaction") occur during drainage, the procedure should be stopped immediately, and the patient should lie flat. If necessary, 0.5 ml of 0.1% adrenaline can be injected subcutaneously, with close monitoring of blood pressure to prevent shock. Generally, there is no need to inject drugs into the pleural cavity after drainage.
Glucocorticoids can reduce hypersensitivity and inflammatory reactions, alleviate toxic symptoms, accelerate effusion absorption, and minimize complications like pleural adhesions or thickening. However, due to potential adverse effects or the risk of {|###|}subcutaneous node{|###|} dissemination, their use should be carefully considered. For acute {|###|}subcutaneous node{|###|} exudative pleuritis with severe systemic toxicity and significant effusion, glucocorticoids (e.g., prednisone or prednisolone 25–30 mg/day, divided into three oral doses) may be added to anti-{|###|}subcutaneous node{|###|} therapy. The dose should be tapered gradually once fever subsides, toxic symptoms improve, and effusion decreases significantly. Abrupt discontinuation should be avoided to prevent rebound effects; the typical course lasts 4–6 weeks.
2. Empyema
Empyema refers to infectious inflammation of the pleural cavity caused by various pathogens, accompanied by turbid, purulent pleural effusion. Bacteria are the most common causative agents. Most bacterial empyemas result from inadequately controlled bacterial pleuritis. A few cases may be caused by {|###|}subcutaneous node{|###|} bacteria, fungi, actinomycetes, or Nocardia. Currently, the most common pathogens in infectious pleural effusions are Gram-negative bacilli, followed by Staphylococcus aureus and pneumococci. Among Gram-negative bacilli, Pseudomonas aeruginosa and {|###|}large intestine{|###|} bacilli are prevalent. Anaerobes are also widely recognized as common pathogens. Empyema secondary to pneumonia is often monomicrobial, whereas cases complicating lung abscess or bronchiectasis are typically polymicrobial. In immunocompromised patients, fungal and Gram-negative bacterial infections are particularly common.
Acute empyema often presents with high fever, wasting, and {|###|}distending pain{|###|} in the chest. The treatment principles include infection control, drainage of pleural effusion, and lung re-expansion to restore pulmonary function. Effective antibiotics should be administered systemically or intrapleurally as early as possible, targeting the causative pathogen. Drainage is the cornerstone of empyema treatment, achieved via repeated aspiration or closed drainage. The pleural cavity may be irrigated with 2% sodium bicarbonate or saline, followed by instillation of antibiotics and streptokinase to thin the pus for easier drainage. A few cases may require intercostal closed drainage with an underwater seal. Patients with bronchopleural fistula should avoid pleural irrigation to prevent bacterial dissemination.
Chronic empyema is characterized by thickening of the pleural membrane, collapse of the thorax, chronic consumption, and clubbing of fingers (toes). Surgical pleural decortication should be considered. In addition, general supportive therapy is also very important. High-energy, high-protein foods containing vitamins, such as common bletilla tuber, should be provided. Correct typical edema, electrolyte disturbances, and maintain acid-base balance. If necessary, small amounts of blood can be transfused multiple times.
III. Malignant Pleural Effusion
Malignant pleural effusion is mostly caused by the progression of malignant tumors and is a common complication of advanced-stage malignancies. For instance, lung cancer accompanied by pleural effusion is already considered advanced stage. Imaging examinations help determine the extent of lesions in the lungs and mediastinal lymph nodes. Given that the pleural fluid grows rapidly and persists, it often leads to severe dyspnea due to compression from large effusions and may even result in death. Therefore, repeated thoracentesis is necessary, but frequent drainage can lead to excessive protein loss (1L of pleural fluid contains 40g of protein), making treatment challenging and often ineffective. Hence, accurate diagnosis of the malignancy and its histological type, along with timely and appropriate treatment, is crucial for symptom relief, pain reduction, improved quality of life, and prolonged survival.
Systemic chemotherapy has some efficacy for pleural effusions caused by certain small-cell lung cancers. Localized radiation therapy may be considered for cases with mediastinal lymph node metastasis. After draining the pleural fluid, intrapleural injection of antitumor drugs such as doxorubicin, cisplatin, fluorouracil, mitomycin, nitrocaphane, and bleomycin is a common treatment method. This helps kill tumor cells, slow the production of pleural fluid, and induce pleural adhesion. Intrapleural injection of biological immunomodulators, such as Corynebacterium parvum vaccine (CP), IL-2, interferon-β, interferon-γ, lymphokine-activated killer cells (LAK cells), and tumor-infiltrating lymphocytes (TIL), is a relatively successful approach explored in recent years for treating malignant pleural effusion. These agents can inhibit malignant cells, enhance local lymphocyte infiltration and activity, and promote pleural adhesion. To obliterate the pleural space, a chest tube can be inserted to drain the fluid completely, followed by injection of pleural adhesives such as tetracycline, erythromycin, or talc powder to induce adhesion between the pleural layers and prevent fluid reaccumulation. Adding small amounts of lidocaine and dexamethasone can alleviate pain and fever-like adverse reactions. Despite these treatments, the prognosis for malignant pleural effusion remains poor.
Pleural fluid examination can generally determine the nature of the effusion. Usually, transudative effusions should prompt a search for systemic factors, while exudative effusions may be related not only to pleural pathology but also to systemic diseases. Differential diagnosis should consider the onset (acute or chronic), fever, weakness, chest pain, and other systemic or localized pulmonary/pleural symptoms; dyspnea, ability to lie flat, presence of lower limb edema; ascites or abdominal masses, superficial lymphadenopathy, joint or skin lesions, etc. This should be combined with corresponding blood tests, chest X-rays, ultrasound, pleural fluid analysis, tuberculin tests, and, when necessary, pleural biopsy for comprehensive analysis.
When diagnosing pleural effusions, the first step is to differentiate between exudative and transudative effusions. The most common cause of exudative pleural effusion is tuberculous pleuritis, which predominantly affects young patients, with positive tuberculin tests. Physical examination typically reveals no significant findings beyond pleural effusion signs. The pleural fluid is straw-colored and lymphocyte-predominant, and pleural biopsy shows no specific changes. Without effective anti-tuberculosis treatment, approximately one-third of patients may develop pulmonary or extrapulmonary tuberculosis within five years. Transudative effusions may be associated with left heart failure, hypoalbuminemia, etc.
Tuberculous and malignant pleural effusions often require careful differentiation, as both are clinically common but have vastly different treatments and prognoses. Malignant pleural effusions, caused by tumor invasion of the pleura, are often bloody, large in volume, rapidly accumulating, and have a pH<7.4,CEA超過10~15μg/L,LDH> >500 U/L, commonly due to lung cancer or breast cancer metastasis to the pleura. Tuberculous pleuritis often presents with fever, pH typically below 7.3, significantly higher ADA activity compared to other causes of effusion, and usually no elevation in CEA or ferritin. If clinical differentiation is difficult, a trial of anti-tuberculosis therapy with monitoring and follow-up may be considered. Elderly patients with tuberculous pleuritis may lack fever, and tuberculin tests are often negative. If tests are negative and anti-tuberculosis therapy is ineffective, malignancy should still be considered. Further differentiation can be aided by pleural fluid cytology, pleural biopsy, chest imaging (CT, MRI), bronchoscopy, and thoracoscopy. CT scans are highly accurate in diagnosing pleural effusions, particularly in distinguishing pleural invasion or widespread metastasis in bronchogenic lung cancer, and are crucial for diagnosing malignant effusions, staging lung cancer, and selecting treatment plans. MRI complements CT in diagnosing pleural effusions, especially malignant ones, with superior characteristic features. Pleural needle biopsy is simple, minimally invasive, and has a diagnostic yield of 40–75%. Thoracoscopy offers the highest diagnostic rate for malignant pleural effusions (70–100%), providing evidence for treatment planning. It allows comprehensive examination of the pleural cavity, observation of lesion morphology, distribution, and adjacent organ involvement, and enables targeted biopsies, resulting in higher diagnostic accuracy and more precise tumor staging. In rare cases where the cause of pleural effusion remains undetermined despite these tests, thoracotomy may be considered if no contraindications exist.






