| disease | Renal Tuberculosis |
Urinary subcutaneous node is secondary to subcutaneous node lesions in other parts of the body, with renal subcutaneous node being the most significant. Among urinary subcutaneous nodes, renal subcutaneous node is the most common and the first to occur, later spreading from the kidney to the entire urinary system. Therefore, renal subcutaneous node essentially represents the significance of urinary subcutaneous node.
bubble_chart Pathogenesis
The pathogenic bacteria of renal subcutaneous nodes primarily originate from pulmonary subcutaneous nodes, but can also come from subcutaneous nodes in bones, joints, intestinal binding nuclei, and other organs. There are four routes by which subcutaneous node bacilli spread to the kidneys: ① Hematogenous dissemination: This is the primary route of infection. Subcutaneous node bacilli invade the bloodstream from pulmonary subcutaneous node lesions and disseminate to the kidneys. ② Urinary tract infection: This is essentially the spread and extension of subcutaneous node bacilli within the urinary system. After subcutaneous node lesions develop in one side of the urinary tract, the bacilli reflux upward from the lower urinary tract to the contralateral kidney. ③ Lymphatic infection: Subcutaneous node bacilli from systemic subcutaneous node lesions or lymphatic subcutaneous node lesions spread to the kidneys via the lymphatic pathways. ④ Direct extension: Subcutaneous node lesions in organs near the kidneys, such as the spine or intestines, directly extend and involve the kidneys.
Extensive experimental studies, autopsies, and clinical observations confirm that hematogenous dissemination is the main mode of infection for renal subcutaneous nodes. Subcutaneous node bacilli invade the kidneys via the bloodstream. When the kidneys exhibit certain susceptibility (e.g., due to local circulatory disturbances, trauma, etc.) or when the number of bacteria increases to form emboli, subcutaneous node lesions first develop in the glomerular capillary tufts of the outer renal cortex. Ninety percent occur in the cortex, while about 10% occur in the medulla. These outer-layer subcutaneous nodes are multifocal and almost always involve both kidneys simultaneously. However, at this stage, due to enhanced systemic immunity and local resistance, as well as reduced bacterial numbers and virulence, the vast majority of cases heal completely without causing any symptoms or being detected. During this period, subcutaneous node bacilli can be detected in the urine, a phase referred to as "pathological renal subcutaneous nodes or preclinical renal subcutaneous nodes." While outer renal lesions persist, if the body's resistance declines, bacterial virulence increases, or local factors worsen, some lesions may fail to heal and progress further, reaching the so-called "clinical stage of renal subcutaneous nodes." About 1–2% of pulmonary subcutaneous node cases develop into this clinical stage. Only 3–7% of outer renal subcutaneous node lesions progress to the clinical stage.
Generally, the progression from asymptomatic preclinical renal subcutaneous nodes to the clinical stage takes a long time. During this period, the primary lesions in the lungs or other organs may have already healed, making it impossible to identify the original source in a significant proportion of renal subcutaneous node patients. If preclinical renal subcutaneous node lesions do not heal, they may spread locally or extend downward along the renal tubules to the inner renal medulla, causing ulcers in the renal papillae. As the lesions expand and rupture, they invade the renal calyces or pelvis. In cases where the inner renal layers are involved, leading to clinical renal subcutaneous nodes, over 85% are unilateral sexually transmitted disease lesions, while bilateral renal subcutaneous nodes account for about 15%. Clinically observed bilateral renal subcutaneous nodes may develop directly from early bilateral sexually transmitted pathological renal subcutaneous nodes, or they may result from apparent healing on one side followed by later progression, or from unilateral clinical renal subcutaneous nodes spreading to the contralateral kidney via urinary tract infection.bubble_chart Pathological Changes
The pathological changes of renal subcutaneous nodules are the same as those of subcutaneous nodules in other organs of the body and can be divided into: ① nodular type; ② ulcerative cavitary type; ③ fibrocalcific type. The early preclinical renal subcutaneous nodule lesions are characterized by foxtail millet-like grayish-white subcutaneous nodules formed by subcutaneous nodule bacilli in the glomeruli. The center of these nodules often undergoes caseous necrosis, surrounded by subcutaneous nodular granulation tissue composed of clusters of epithelioid cells interspersed with a few multinucleated giant cells (Langhans giant cells), lymphocytes, and fibroblasts. If the lesions fail to heal and instead expand and spread, they progress to the clinical stage of renal subcutaneous nodules.
The pathological changes of clinical-stage renal subcutaneous nodules involve the gradual extension of foxtail millet-like subcutaneous nodules from the glomeruli to the renal papillae, where they ulcerate. Subsequently, the lesions involve the renal calyceal membrane, forming irregular ulcers. The disease can spread directly to distant areas via the renal calyces and pelvis, or the subcutaneous nodule bacilli can disseminate through the renal lymphatic channels to involve the entire kidney. When the caseous necrotic material in the center of the subcutaneous nodules in the renal papillae liquefies and drains into the renal pelvis, subcutaneous nodular cavities form. These cavities may be confined to a part of the kidney or involve the entire kidney, resulting in a "subcutaneous nodular pyonephrosis." This type of pathological change is the most common clinically. In some cases, if the body's resistance is enhanced, the caseous material may become concentrated without liquefying, leading to extensive fibrous tissue proliferation and calcification, clinically referred to as "autoamputated kidney" or "mortar kidney." Although the disease may clinically progress to the calcified autoamputation stage, the actual pathology often involves a mixture of caseous cavities, fibrous atrophy, induration, and calcification, with subcutaneous nodule bacilli potentially still present in the caseous material.Renal subcutaneous nodule lesions can spread directly downward, or the subcutaneous nodule bacilli from the lesions can disseminate via urine to involve the ureteral membrane, submucosa, or even the muscular layer, causing subcutaneous nodules, caseous necrosis, and ulcers. Subsequently, fibrous tissue proliferation occurs, leading to partial occlusion of the ureteral lumen, uneven thickness, and a rough inner surface. In extensive cases, the entire ureter may be affected, becoming a rigid cord, shortening the ureter and causing the ureteral orifice in the bladder to retract upward and inward, forming a concave cavity.
During the clinical stage of renal subcutaneous nodules, when the ureter is involved but the lumen remains patent and not yet occluded, direct spread of subcutaneous nodule lesions or contact dissemination of subcutaneous nodule bacilli within the bladder can lead to bladder subcutaneous nodules. Initially, this causes mucosal congestion and edema, followed by the formation of subcutaneous nodules or ulcers. These early bladder lesions are often localizedto the area around the ureteral orifice on the same side as the renal lesion, later spreading to the entire bladder. If the disease progresses, it may invade the muscular layer, causing fibrosis of the bladder tissue, leading to loss of elasticity, reduced capacity, and ultimately bladder contracture. This can also involve the contralateral ureter and kidney, resulting in hydronephrosis.
bubble_chart Clinical Manifestations
Renal subcutaneous nodules mostly occur in adults. According to comprehensive statistics in China, 75% of cases occur between the ages of 20 and 40, but they can also occur in childhood and old age. The incidence is slightly higher in males than in females. The clinical manifestations of renal subcutaneous nodules vary depending on the location of the lesion and the extent of tissue damage. In the {|initial stage [first stage]|} when the lesion is confined to a specific part of the kidney, clinical symptoms are minimal, with abnormalities only detected during urine tests. Subcutaneous nodule bacilli can be found in the urine. When subcutaneous nodules spread from the kidney to affect the bladder, causing bladder subcutaneous nodules, a series of symptoms emerge, including:
(1) Bladder irritation symptoms Bladder irritation symptoms are the most important, primary, and earliest symptoms of renal subcutaneous nodules. When subcutaneous nodule bacilli cause subcutaneous nodule-induced inflammation in the bladder mucosa, patients initially experience frequent urination, with the number of urinations gradually increasing both during the day and night, ranging from several times a day to dozens of times. In severe cases, patients may urinate multiple times per hour, even to the point of resembling urinary incontinence. Approximately 75–80% of patients exhibit frequent urination. Along with frequent urination, urgency and dysuria may occur, making it difficult to delay urination and causing a burning pain in the urethra or suprapubic bladder area at the end of urination. As bladder lesions worsen, these symptoms become more pronounced.
(2) Hematuria Hematuria is the second most important symptom of renal subcutaneous nodules, occurring in about 70–80% of cases. It usually appears alongside symptoms such as frequent urination, urgency, and dysuria. Most hematuria originates from bladder lesions, but it can also stem from the kidney itself. The severity of hematuria varies, mostly presenting as grade I gross hematuria or microscopic hematuria. However, in 3% of cases, significant gross hematuria is the sole initial symptom.
Most cases of hematuria are terminal hematuria, caused by bleeding due to bladder contraction during urination in subcutaneous nodule-induced inflammation and ulcers. If hematuria originates from the kidney, it may present as total hematuria.
(3) Pyuria Due to subcutaneous nodule-induced inflammation in the kidneys and bladder, tissue destruction occurs, leading to a large number of pus cells in the urine. The urine may also contain caseous material, making it turbid. In severe cases, the urine resembles rice-water-like pyuria. The incidence of pyuria is around 20%.
(4) Lumbago Severe renal subcutaneous nodule lesions can cause subcutaneous nodule-induced pyonephrosis, enlarging the kidney and forming a mass in the lumbar region, leading to lumbago. Domestic data indicate an incidence rate of 10%. If contralateral hydronephrosis occurs, lumbar symptoms may also appear on the opposite side. In rare cases, blood clots or pus passing through the ureter can cause renal colicky pain.
(5) Systemic symptoms Since renal subcutaneous nodules are part of systemic subcutaneous nodule disease, general symptoms of subcutaneous nodule lesions may appear, such as loss of appetite, weight loss, lack of strength, night sweats, and low-grade fever. These symptoms may arise when renal subcutaneous nodules become severe or due to subcutaneous nodules in other organs.
(6) Other symptoms Because renal subcutaneous nodules may be secondary to or complicated by subcutaneous nodules in other organs, symptoms of subcutaneous nodules in other organs may appear, such as cold abscesses in bone subcutaneous nodules, sinuses in lymph subcutaneous nodules, diarrhea and abdominal pain in intestinal binding nodules, and nodules in the epididymis when male genital tract subcutaneous nodules are present.
The pathological process of renal subcutaneous node is very slow, and its clinical manifestations are mainly bladder irritation symptoms. Therefore, the diagnosis of renal subcutaneous node is based on the symptoms of cystitis (frequency, urgency, and dysuria). Except when there is an obvious cause of cystitis, the possibility of renal subcutaneous node should be considered, and further systematic examinations are necessary.
(1) History analysis and physical examination Long-term chronic frequency, urgency, dysuria, and hematuria, or cystitis that does not respond to general anti-inflammatory treatment, should all raise suspicion of renal subcutaneous node lesions. Especially in young adult males with urinary tract infections and no growth of common bacteria in urine cultures, urological subcutaneous node examinations should be performed. During physical examination, attention should be paid to systemic subcutaneous node lesions, particularly in the male reproductive tract, checking the prostate, vas deferens, and epididymis for nodules. In the urinary system, the renal area should be examined for masses, and the costovertebral angle for tenderness.
(2) Laboratory tests
1. Routine urine examination Urine is often acidic, containing small amounts of protein, and microscopic examination in most patients reveals small to moderate amounts of red and white blood cells. However, in cases of mixed urinary tract infections, the urine may be alkaline, with large numbers of white blood cells or pus cells under the microscope.
2. Routine urine bacterial culture Renal subcutaneous node is a specific infection of the urinary system. Routine urine bacterial cultures should be negative. However, a significant proportion of renal subcutaneous node patients have mixed urinary tract infections, and routine urine bacterial cultures may be positive. Reports indicate that 1/3 to 1/2 of renal subcutaneous node cases are accompanied by mixed urinary tract infections.
3. Urine subcutaneous node bacillus examination
(1) 24-hour urine acid-fast bacillus examination Subcutaneous node bacillus is a type of acid-fast bacillus. Concentrated 24-hour urine samples are directly smeared and stained for acid-fast bacillus examination. This method is simple, yields rapid results, and has a positivity rate of 50–70%. However, smegma bacillus and Mycobacterium phlei are also acid-fast bacilli commonly found in urine. Therefore, the presence of acid-fast bacilli in urine does not necessarily indicate subcutaneous node bacillus. Nevertheless, repeated findings of the same acid-fast bacilli, combined with clinical history and characteristics, can still provide some diagnostic reference for renal subcutaneous node.
(2) Urine subcutaneous node bacillus culture Urine subcutaneous node bacillus culture is definitive for diagnosing renal subcutaneous node. A positive culture confirms the diagnosis. However, the process is time-consuming, taking 1–2 months, with a positivity rate as high as 90%.
(3) Urine subcutaneous node bacillus animal inoculation The results of urine subcutaneous node bacillus animal inoculation are highly valuable for diagnosing renal subcutaneous node and can serve as a basis for diagnosis, with a positivity rate exceeding 90%. However, this method is also time-consuming, requiring up to 2 months for results.
4. Urine subcutaneous node IgG antibody measurement Nassau et al. found that active subcutaneous node patients produce a certain amount of specific antibodies. Grauge et al. demonstrated that these specific antibodies belong to the IgG class. The First Affiliated Hospital of Hubei Medical University reported using polymerized OT as an antigen and enzyme-linked immunosorbent assay (ELISA) to measure urine subcutaneous node IgG antibodies. Renal subcutaneous node patients had subcutaneous node IgG antibodies in their urine, with a positivity rate of 89.1%. This test has certain specificity and sensitivity, providing significant clinical value for diagnosing renal subcutaneous node. However, in advanced-stage renal subcutaneous node with severe renal impairment preventing urine secretion, or when renal subcutaneous node is complicated by ureteral obstruction and urine from the affected side cannot be excreted (with urine samples coming from the healthy kidney), false negatives may occur.
5. Subcutaneous node bacillus test The subcutaneous node bacillus test is a method to determine whether a person is infected with subcutaneous node bacillus. It is most commonly used for pulmonary subcutaneous node disease but also has reference value for subcutaneous node lesions in other organs.
(1) There are several types of subcutaneous node bacteria: ① old subcutaneous node bacteria; ② pure subcutaneous node bacteria; ③ pure protein derivatives made from atypical mycobacteria; ④ four types of carrageenan. Generally, the old subcutaneous node bacteria are used for testing.
(2) Preparation of old tuberculin (OT): Culture human-type tubercle bacilli for 2 months, inactivate by heating, filter out dead bacteria, and evaporate to concentrate to 1/10 of the original volume to obtain the tuberculin stock solution. Subsequently, according to the 1952 World Health Organization standards, each milliliter contains 10 tuberculin units (TU), equivalent to 1000 mg.
(3) Test method: Use standardized old tuberculin solution. For the first test, inject 0.1 ml of a 1/1000 or 1/2000 dilution (containing 10.5 TU per 0.1 ml) intradermally into the middle third of the inner side of the left forearm. Observe the reaction after 48–72 hours. If negative, repeat the test with a 1/100 dilution (containing 100 TU per 0.1 ml) and evaluate the reaction result.
(4) Positive criteria for the tuberculin test (Table 1):
Table 1 Positive Criteria for the Tuberculin Test
| Diameter of local induration on forearm | Reaction | Symbol |
| <5 mm | Negative | - |
| 5–10 mm | Positive | + |
| 11–20 mm | Positive | ++ |
| >20 mm | Strongly positive | +++ |
| Local blistering or necrosis | Strongly positive | ++++ |
(5) Significance of a positive tuberculin reaction: ① Artificial immunization due to BCG vaccination. ② Infection with tubercle bacilli, but further confirmation or exclusion of active tuberculosis is needed. ③ Significance in children: - Under 8 years old: >50% likelihood of active tuberculosis. - Under 4 years old: Almost all cases suggest possible active tuberculosis. - Under 3 years old: Not only active tuberculosis but also poor prognosis if untreated. - Under 1 year old: Definitive active tuberculosis with poor prognosis if untreated. ④ A strongly positive tuberculin test indicates active tuberculosis and requires medical evaluation.
6. Erythrocyte sedimentation rate (ESR) test: Renal tuberculosis is a chronic, long-term condition and a consumptive disease, so the ESR may be elevated. Li Zhe reported that among 300 cases of renal tuberculosis, 255 showed an increased ESR. However, the ESR test is not specific for renal tuberculosis. Nevertheless, in patients with cystitis accompanied by an elevated ESR, it often suggests possible renal tuberculosis, making it a useful reference test.
7. Renal function tests:
(1) Measurement of blood urea nitrogen (BUN), creatinine, and uric acid: Unilateral renal tuberculosis does not affect renal function tests. However, if severe renal tuberculosis affects the contralateral kidney or causes hydronephrosis, these tests may show elevated values. Although renal function tests are not direct diagnostic indicators for renal tuberculosis, they provide crucial reference values for managing renal tuberculosis patients and must be routinely performed.
(2) Radionuclide renogram examination: If the kidney lesion is localized and does not impair the secretory function of the entire kidney, the renogram will appear normal. If a significant portion of the kidney parenchyma is damaged, the renogram may show insufficient blood supply or prolonged secretion and excretion times. In cases of severe kidney damage, the renogram will display a flat, non-functional line. If a renal subcutaneous node causes contralateral hydronephrosis, the renogram may reveal a hydronephrotic or obstructive curve. Although this test lacks specific diagnostic value, it is simple to perform and causes no discomfort to the patient, making it a routine clinical examination method.
(3) Cystoscopy Cystoscopy is an important diagnostic method for renal tuberculosis. It allows direct visualization of typical tuberculous changes in the bladder, thereby confirming the diagnosis. Early bladder tuberculosis may show congestion, edema, and tuberculous nodules on the bladder mucosa, often concentrated around the ureteral orifice on the same side as the renal lesion, later spreading to the trigone and other areas. Severe bladder tuberculosis may exhibit widespread mucosal congestion, edema, tuberculous nodules, and ulcers, with the ureteral orifice retracting upward into a cave-like appearance. By intravenously injecting indigo carmine and observing the timing of blue dye excretion from both ureteral orifices, the renal function of each side can be assessed. During cystoscopy, bilateral retrograde catheterization can also be performed, inserting ureteral catheters into both renal pelvises to collect urine for microscopic examination, tuberculous bacilli culture, and animal inoculation. Since these are split renal function tests, their diagnostic value is particularly significant. After retrograde catheterization, contrast agents (e.g., 12.5% sodium iodide or meglumine diatrizoate) can be injected into the bilateral ureteral catheters for retrograde pyelography to evaluate both kidneys. Most patients can determine the nature, location, and severity of the lesions. If bladder tuberculosis is severe, with bladder contracture and a capacity of less than 100 ml, visualization of the bladder interior becomes difficult, and this examination should not be performed.
(4) X-ray Examination X-ray examination is the primary diagnostic method for renal tuberculosis. A typical tuberculous image on X-ray can confirm the diagnosis of renal tuberculosis. Routine X-ray examinations include the following:
1. Plain Radiography of the Urinary Tract The plain film may show an enlarged or lobulated kidney. In 4.5–31% of cases, patchy, cloud-like, or plaque-like calcifications of renal tuberculosis can be observed. These calcifications are irregularly distributed and amorphous, usually limited to one kidney. If calcification involves the entire tuberculous kidney or even the ureter, it forms the so-called "autonephrectomy" (Figure 1).
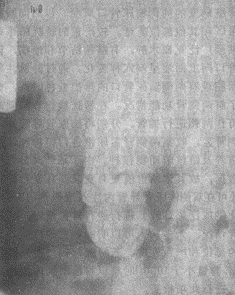
Figure 1 Plain Radiograph of Renal Tuberculosis "Autonephrectomy" Showing Calcification Involving the Entire Tuberculous Kidney
2. Intravenous Pyelography (IVP) IVP, also known as excretory or descending urography, involves injecting a contrast agent intravenously, which is then excreted by the kidneys. X-ray images are taken when the contrast fills the renal calyces and pelvis. Commonly used contrast agents include meglumine diatrizoate (Urografin), sodium diatrizoate (Hypaque), and iodopyracet (Diodrast). Currently, non-ionic contrast agents such as Iopamiro, Omipaque, and Ultravist are widely used, significantly reducing iodine toxicity and adverse reactions. Since the contrast agent is excreted by the kidneys to visualize the urinary system, this method not only identifies renal lesions but also assesses renal function. Typical tuberculous manifestations include destruction of the renal parenchyma. Lesions confined to the renal papillae and minor calyces appear with irregular, moth-eaten edges (Figure 2). The infundibulum may become deformed, shrunken, or disappear due to inflammatory lesions or scar contraction. In extensive cases, complete destruction of the calyces may occur, with caseous necrosis forming irregular-edged "cotton-ball-like" tuberculous cavities (Figure 3). If the entire kidney is destroyed, forming a pyonephrosis with loss of function, the affected kidney will not be visualized on IVP. Ureteral tuberculosis on X-ray may show irregular walls, uneven lumen diameter, and loss of normal flexibility, appearing as a rigid, straight tube.
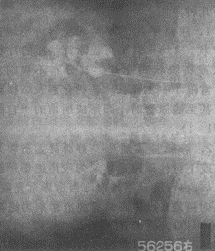
Figure 2 Renal Tuberculosis Showing Moth-Eaten Appearance of the Renal Calyx
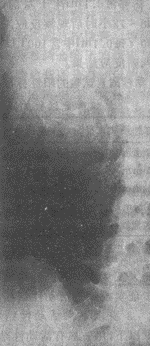
Figure 3 Kidney subcutaneous nodule Renal calyx caseous necrosis and cavitary changes
3. High-dose intravenous pyelography: If the patient's overall renal function is poor and conventional intravenous pyelography fails to adequately visualize the kidneys, a high-dose intravenous pyelography with an increased amount of contrast agent may be performed. This can make previously unclear lesions appear clearly. The commonly used method involves administering 2 ml of 50% diatrizoate meglumine contrast agent per kilogram of body weight, mixed with an equal volume of 5% glucose solution or normal saline, via rapid intravenous drip over 5–8 minutes. Fasting from water before the procedure is unnecessary, and ureteral compression is not required during the examination. However, the total volume of contrast agent should not exceed 140 ml.
4. Retrograde pyelography: After inserting a ureteral catheter into the renal pelvis via cystoscopy, contrast agent is injected retrogradely through the catheter into the renal pelvis for X-ray imaging, a procedure known as retrograde pyelography. Typically, a 12.5% iodine-based contrast agent is used; if the patient is allergic to iodine, 12.5–25% sodium bromide may be substituted. Since the concentration and volume of the injected contrast agent can be adjusted as needed, renal lesions can be visualized more clearly, thereby improving diagnostic accuracy. This method is suitable when intravenous pyelography cannot be performed or yields unsatisfactory results. However, unlike intravenous pyelography, it does not provide information about changes in renal function.
5. Antegrade pyelography via renal pelvis puncture: For cases where neither intravenous nor retrograde pyelography can be performed, or when the nature of the lesion remains unclear, direct renal pelvis puncture followed by contrast agent injection may be conducted. This can reveal typical X-ray findings of renal subcutaneous nodules or other lesions, playing a decisive role in diagnosis. After the puncture, the aspirated renal contents can also undergo various laboratory tests and subcutaneous nodule bacterial examinations. With advancements in ultrasound technology, guided renal pelvis puncture has become safer and more accurate.
bubble_chart Treatment Measures
Renal subcutaneous node is secondary to systemic subcutaneous node disease, so in treatment, systemic therapy must be emphasized and combined with consideration of local lesions to achieve relatively satisfactory results.
(1) Systemic therapy Systemic therapy includes appropriate rest, medical physical activities, adequate nutrition, and necessary drug treatment (including measures to treat systemic subcutaneous node lesions other than renal subcutaneous node).
(2) Drug treatment Due to significant differences in the scope of local lesions and the degree of destruction in renal subcutaneous node, treatment targeting local lesions varies from case to case. Before the discovery of anti-subcutaneous node drugs such as streptomycin, the only treatment for clinically confirmed renal subcutaneous node was nephrectomy. After the 1940s, streptomycin and para-aminosalicylic acid were successively introduced, and many clinical cases of renal subcutaneous node could be cured with drug treatment alone. After the 1950s, the emergence of isoniazid, which is highly effective, low in toxicity, and inexpensive, combined with the use of multiple drugs, greatly improved the efficacy of renal subcutaneous node treatment, almost curing all early subcutaneous node lesions. By 1966, rifampin was clinically applied, and due to its significant efficacy and few side effects, its use in combination with other drugs further enhanced the treatment outcomes for renal subcutaneous node. Currently, the number of cases requiring nephrectomy due to renal subcutaneous node has significantly decreased. However, in some regions with poor sanitary conditions and inadequate medical resources, cases of renal subcutaneous node still occur, and even some advanced-stage patients are discovered. For patients diagnosed with renal subcutaneous node, regardless of the severity of the lesions or the need for surgical intervention, anti-subcutaneous node drugs must be taken according to a specific regimen.
1. Indications for the use of anti-subcutaneous node drugs
(1) Preclinical renal subcutaneous node.
(2) Unilateral or bilateral renal subcutaneous node confined to one group of major calyces.
(3) Solitary kidney with renal subcutaneous node.
(4) Cases with active subcutaneous node in other parts of the body where renal subcutaneous node surgery is temporarily unsuitable.
(5) Bilateral grade III renal subcutaneous node where surgery is not advisable.
(6) Renal subcutaneous node complicated by other severe diseases where surgery is temporarily unsuitable.
(7) As preoperative medication in conjunction with surgical treatment.
(8) Routine medication after renal subcutaneous node surgery.
2. Common types of anti-subcutaneous node drugs Since various anti-subcutaneous node drugs have their pharmacological characteristics, the requirements and precautions for their use also differ. The commonly used anti-subcutaneous node drugs are briefly introduced as follows:
(1) Streptomycin: It has bactericidal effects on subcutaneous node bacilli, with efficacy at a concentration of 1.0μg/ml. The peak serum concentration occurs 1 hour after intramuscular injection, decreasing by 50% after 3 hours, and about 60–90% is excreted through the kidneys in urine. Its bacteriostatic effect is strongest at pH 7.7–7.8 and significantly weaker below 5.5–6.0. Concurrent administration of sodium bicarbonate to alkalinize the urine can enhance its efficacy. The general adult dosage is 1.0g daily, divided into two intramuscular injections; when used in combination with other anti-subcutaneous node drugs, the dosage is 2g weekly or 1g every 3 days. Streptomycin treatment can lead to fibrosis of subcutaneous node lesions. If the lesions are located in the urinary excretory system, such as the ureter, local fibrotic contraction may cause obstruction, which should be noted. Perioral numbness may occur after streptomycin injection, and if not severe, treatment can continue, as it often gradually diminishes with use. The main side effect is its impact on the vestibular branch of the eighth cranial nerve. A few cases may experience anaphylactic shock.
(2) Isoniazid (INH, Rimifon): It has inhibitory and bactericidal effects on subcutaneous nodule bacilli. A daily dose of 200–300 mg can achieve satisfactory bactericidal concentrations. Peak serum concentration is reached 1–2 hours after oral administration. The half-life is 6 hours, and effective bacteriostatic concentrations can still be detected in the blood after 24 hours. The recommended dosage is generally 300 mg per day, taken in a single dose. This dosage rarely causes adverse reactions, allowing for long-term use, even for several years. After ingestion, isoniazid is rapidly absorbed and penetrates tissues, easily infiltrating fibrotic and caseous lesions. It promotes vascular regeneration in subcutaneous nodule lesions, facilitating the entry of anti-subcutaneous nodule drugs into the affected areas. Its main side effects include mental excitation and polyneuritis, which are believed to be related to increased vitamin B6 excretion or interference with pyridoxine metabolism. Therefore, vitamin B6 (5–10 mg) should be co-administered with isoniazid to prevent these side effects. Serum transaminase levels may rise during medication, but this does not cause liver damage.
(3) Para-aminosalicylic acid (PAS): It has a bacteriostatic effect on subcutaneous node bacilli. The plasma concentration peaks 1-2 hours after administration, and only trace amounts remain in the blood after 4-6 hours. The daily dose is 8-12g, divided into 3-4 doses. This drug is less effective when used alone but can enhance the anti-subcutaneous node bacilli effects of streptomycin and isoniazid, as well as delay the development of drug resistance. Therefore, clinically, combining two or three anti-subcutaneous node drugs is beneficial for maximizing therapeutic efficacy. The main side effects include gastrointestinal reactions such as nausea, vomiting, and diarrhea, which is why it is increasingly being replaced by rifampin and ethambutol. This drug should not be used in combination with rifampin.
(4) Rifampin (Rifampin, RFP): A semi-synthetic, broad-spectrum oral antibiotic with strong bactericidal effects against both intracellular and extracellular actively growing subcutaneous node bacilli. It is more potent than streptomycin, para-aminosalicylic acid, and ethambutol, and is also effective against drug-resistant subcutaneous node bacilli. The drug concentration peaks 2-4 hours after administration, and serum levels remain relatively high even after 12 hours. The daily dosage is 600-900mg, taken once or twice on an empty stomach. It has no cross-resistance with other anti-subcutaneous node drugs and can enhance the effects when combined with isoniazid or ethambutol. Side effects are rare but may include occasional digestive tract reactions and rashes. In recent years, a few cases of liver damage, elevated serum transaminase levels, and jaundice have been reported.
(5) Ethambutol (EMB): It has a bacteriostatic effect on all types of subcutaneous node bacilli. The plasma concentration peaks 2-4 hours after oral administration, with 50% excreted via the kidneys within 24 hours and a small portion excreted in feces. There is no accumulation in individuals with normal kidney function. The drug is well absorbed and has good tissue penetration, even reaching caseous fibrous lesions. Its primary toxicity involves retrobulbar neuritis, leading to symptoms such as blurred vision, color blindness (especially for green), or narrowed visual fields, and in severe cases, blindness. Optic neuritis is reversible and often resolves after discontinuation. The incidence of toxic reactions is dose-dependent. The usual daily dose is 600mg, taken once or divided into three doses, with fewer toxic reactions observed within this range. Regular vision and color discrimination tests should be conducted during treatment.
(6) Kanamycin: A broad-spectrum antibiotic with primarily bacteriostatic effects against subcutaneous node bacilli. It is poorly absorbed orally and is typically administered intramuscularly at 0.75-1.0g daily. The peak blood concentration occurs 30-60 minutes after injection and lasts for about 6 hours, with approximately 90% excreted in urine within 24 hours. It remains effective against subcutaneous node bacilli resistant to streptomycin, isoniazid, and para-aminosalicylic acid. However, using it alone can easily lead to drug resistance. There is unidirectional cross-resistance with streptomycin: streptomycin-resistant strains may still be sensitive to kanamycin, but kanamycin-resistant strains are not sensitive to streptomycin. Therefore, it should only be considered when streptomycin is contraindicated or when the subcutaneous node bacilli have developed resistance. Its toxic effects primarily involve damage to the eighth cranial nerve, potentially causing permanent deafness, as well as degenerative changes in nerve fibers. It can also cause grade I kidney damage, with tubular proteinuria observed.
(7) Cycloserine (Seromycin): It has a broad antibacterial spectrum and exhibits bacteriostatic effects against subcutaneous node bacilli. However, it is only effective against human subcutaneous node disease and has limited efficacy against animal subcutaneous node disease or in vitro cultures. It is effective against subcutaneous node bacilli resistant to isoniazid, streptomycin, and para-aminosalicylic acid. Its efficacy is comparable to para-aminosalicylic acid but inferior to streptomycin. The oral dose should not exceed 500mg daily and is usually combined with isoniazid and streptomycin. Side effects are severe, primarily affecting the central nervous system, including dizziness, depression, convulsions, and epileptic seizures.
(8) Pyrazinamide (PZA): It is an old drug with new applications. After the 1970s, it was discovered that oral administration produces pyrazinoic acid, which is effective against Mycobacterium tuberculosis and can kill stubborn bacteria deeply hidden within cells. Drug resistance develops rapidly, usually occurring within 1-3 months of use. When combined with rifampin and isoniazid, it can shorten the treatment course. The side effect is hepatotoxicity, which in severe cases may cause acute yellow liver atrophy. The usual dose is 1.5-2.0g per day.
In addition to the aforementioned drugs, there are viomycin and ethionamide (1314), with a dosage of 0.5–0.8 g per day, administered in 2–3 divided doses. The daily dosage of P-acetyl aminobenzaldehyde, thiosemicarbazone (TB1), is 500 mg, taken orally in two divided doses. Capromycin and subcutaneous node antibiotics such as actinomycin may also be considered for use when necessary.
3. Methods of Using Anti-subcutaneous node Drugs In the early stages of clinical application of anti-subcutaneous node drugs, monotherapy was generally employed. However, current practice advocates the combined use of two or more anti-subcutaneous node drugs. The primary drawback of monotherapy is the increased likelihood of developing drug resistance and toxic reactions. Combining two drugs can delay the emergence of resistance by twofold, while using three drugs can extend this delay by three to four times.
(1) Selection and Combination of Anti-subcutaneous node Drugs There is a wide variety of anti-subcutaneous node drugs, with the ideal ones being those that are sensitive to subcutaneous node bacilli, achieve sufficient bacteriostatic or bactericidal concentrations in the blood, and are well-tolerated by the body. Previously, streptomycin and isoniazid were considered first-line drugs, while para-aminosalicylic acid was classified as second-line, and others as third-line. First- and second-line drugs were the preferred choices, with third-line drugs reserved for cases where first- or second-line drugs were ineffective or resistance developed. However, recent in-depth studies and efficacy observations have reclassified isoniazid, rifampin, pyrazinamide, and streptomycin as first-line anti-subcutaneous node drugs. Isoniazid is highly effective against subcutaneous node bacilli, killing both intracellular and extracellular bacteria, and can penetrate caseous lesions and macrophages. Rifampin rapidly kills dividing subcutaneous node bacilli and can enter renal cavities and macrophages. Pyrazinamide exhibits stronger bactericidal effects in acidic environments and can penetrate macrophages, where the low pH enhances its antibacterial action. Streptomycin is highly effective against actively dividing subcutaneous node bacteria and can infiltrate subcutaneous node abscesses.
Regarding the specific application of anti-subcutaneous node drugs, current practice involves combining two or three drugs. Internationally, the era of using streptomycin, isoniazid, and para-aminosalicylic acid as the primary anti-subcutaneous node drugs is gradually fading, replaced by newer drugs and combinations. Domestically, these three drugs are still commonly used, though there is a trend toward replacing them with rifampin. However, isoniazid remains a cornerstone of anti-subcutaneous node therapy. Common combinations now include isoniazid and rifampin, or rifampin and ethambutol. Triple-drug regimens such as streptomycin, rifampin, and pyrazinamide; isoniazid, streptomycin, and rifampin; isoniazid, streptomycin, and ethambutol; or isoniazid, rifampin, and ethambutol are also frequently selected in clinical practice.
(2) Duration of Anti-subcutaneous node Drug Therapy With the continuous emergence of new and effective anti-subcutaneous node drugs, clinical treatment methods have significantly evolved. To achieve optimal therapeutic outcomes, five principles must be adhered to: early intervention, combination therapy, adequate dosage, sufficient duration, and regular administration. Current treatment regimens include the following:
Long-term therapy: Regarding the duration of anti-subcutaneous node drug application, both domestic and international practices predominantly adopt long-term therapy, involving continuous medication for 18 to 24 months, with a minimum of one year. This method is widely recognized for its reliable efficacy and low recurrence rates. According to Lattimer's renal subcutaneous node classification for anti-subcutaneous node drug treatment, the clinical pre-stage renal subcutaneous node requires a one-year medication course. A typical subcutaneous node lesion in a single renal calyx necessitates two years of medication, while lesions in three renal calyces or more extensive subcutaneous node pathologies require over three years of treatment. Petkovio advocates a two-year treatment period for unilateral renal subcutaneous node, with longer medication durations yielding better outcomes for bilateral renal subcutaneous node cases, hence recommending 4 to 5 years, or even over 6 years. Currently, Toman proposes a "two-phase therapy regimen" comprising rifampin and ethambutol. The initial intensive phase lasts 1 to 3 months, combining isoniazid, rifampin, and ethambutol or streptomycin. The late stage [third stage] is the continuation phase, lasting 4 to 12 months, involving a combination of isoniazid and rifampin or ethambutol. This approach significantly enhances efficacy, achieving excellent results even with a medication period of less than 12 months. The main drawback of long-term therapy is the extended duration, leading to poor patient adherence, with issues like irregular dosing, overuse, or misuse, resulting in drug resistance, reduced efficacy, persistent positive urine subcutaneous node bacilli, or recurrence after subcutaneous node control. Domestic reports indicate a 90.3% success rate for regular anti-subcutaneous node treatment, compared to 43.7% for irregular treatment.
Short-course therapy: The primary goal of short-course treatment is to rapidly eliminate subcutaneous node bacilli in the lesions of subcutaneous node, enabling tissue repair and achieving lasting clinical cure. In recent years, new anti-subcutaneous node bactericidal drugs have emerged, making short-course anti-subcutaneous node treatment feasible. Research on short-course drug therapy for renal subcutaneous node began in 1970, and by 1977, studies by Gow et al. laid the foundation for establishing short-course treatment regimens. Currently, the short-course regimen lasts 4 months. In the first two months, pyrazinamide is administered at 25mg/(kg·d) (maximum daily dose of 2g), isoniazid at 300mg/d, and rifampin at 450mg/d. For severe kidney and bladder lesions, streptomycin may be added via intramuscular injection at 1g daily. In the subsequent two months, isoniazid is given at 600mg three times a week, and rifampin at 900mg three times a week. Gow reported that among 140 cases, all were cured except for one case of recurrence due to irregular medication. Urinary subcutaneous node bacilli turned negative after two months of treatment, with mild drug toxic reactions. However, it should be noted that isoniazid, rifampin, and pyrazinamide all have hepatotoxic effects. Treatment should be discontinued if jaundice or elevated transaminase levels occur and resumed only after normalization. Dutt and Sfead used a 9-month short-course regimen with isoniazid and rifampin. In the first month, isoniazid 300mg and rifampin 600mg were administered once daily; for the next 8 months, isoniazid 900mg and rifampin 600mg were given twice weekly, achieving excellent results. In summary, for short-course therapy to succeed, at least two bactericidal drugs (e.g., isoniazid and rifampin) must be combined with one semi-bactericidal drug (e.g., pyrazinamide or streptomycin). The advantages of short-course therapy include: ① Treatment duration is halved or more compared to long-course therapy. ② Total drug usage is reduced. ③ Risk of chronic drug poisoning is minimized. ④ Costs are saved. ⑤ Better patient compliance and adherence to medication schedules are achieved.
Since the growth and reproduction of subcutaneous node bacilli follow a specific pattern, taking 13/4 to 31/2 days, and their growth is inhibited upon exposure to anti-subcutaneous node drugs (e.g., streptomycin, pyrazinamide, rifampin), which can delay the growth phase by 8–10 days, 5–10 days, and 2–3 days, respectively, intermittent dosing of anti-subcutaneous node drugs can be employed based on these characteristics. By spacing doses more than one day apart, similar efficacy to continuous long-course therapy can be achieved. In practice, long-course therapy is typically followed for the first three months, after which intermittent dosing is adopted while maintaining the same total drug dosage as long-course therapy. This approach reduces side effects and improves therapeutic outcomes.
(3) Criteria for Discontinuing Anti-Subcutaneous Node Drugs During anti-subcutaneous node drug therapy, close monitoring of disease progression and regular relevant examinations are essential. Once the lesions are confirmed to have healed, discontinuation of medication may be considered. The current criteria for stopping treatment are as follows:
A. Significant improvement in overall condition, normal ESR, and normal body temperature.
B. Complete resolution of urinary symptoms.
C. Repeated routine urine tests show normal results.
D. Long-term repeated 24-hour urine concentration tests for acid-fast bacilli are consistently negative.
E. Urinary subcutaneous node culture and animal inoculation tests for subcutaneous node bacilli are negative.
F. X-ray urography shows stable or healed lesions.
G. Comprehensive examination reveals no other subcutaneous node lesions elsewhere in the body.
After discontinuation of medication, patients must be advised to undergo long-term follow-up, including regular urine tests and urography for at least 3–5 years.
(3) Surgical Treatment Although anti-subcutaneous node drug therapy can currently control and cure most patients with renal subcutaneous node, there are still some patients for whom medication is ineffective and surgical treatment is still required. Surgical options include total nephrectomy, partial nephrectomy, and renal lesion removal, depending on the extent of the lesion, degree of damage, and the response to drug therapy.
1. Total Nephrectomy
(1) Indications for total nephrectomy: ① Unilateral renal subcutaneous node lesions with extensive destruction exceeding 50%. ② Complete loss of renal function due to subcutaneous node destruction. ③ Subcutaneous node-induced pyonephrosis. ④ Bilateral renal subcutaneous node, with severe destruction on one side and grade I subcutaneous node on the other; the severely affected side is removed, while the grade I side is treated with medication. ⑤ Autonephrectomy (calcified putty kidney).
(2) Anti-subcutaneous node drug application before and after nephrectomy: Since renal subcutaneous node is part of systemic subcutaneous node disease, a secondary subcutaneous node, and a component of urinary tract subcutaneous node, surgical injury during nephrectomy can reduce the body's resistance, potentially activating or spreading subcutaneous node lesions outside the kidney. Therefore, anti-subcutaneous node drugs must be used before and after nephrectomy to control the condition.
1) Preoperative preparation with anti-subcutaneous node drugs: The types and doses of anti-subcutaneous node drugs used for preoperative preparation are the same as in general anti-subcutaneous node therapy. However, the administration methods and duration differ. For example: - Isoniazid 100mg orally three times daily. - Streptomycin 0.5g intramuscularly twice daily. - Rifampin 300mg orally twice daily. This regimen is administered daily for 2 weeks before surgery. If the patient's overall condition is poor or other organ subcutaneous node is present, preoperative drug preparation may be extended, sometimes to 3–4 months. Postoperatively, the same regimen continues until physical recovery (approximately 2 weeks), after which conventional anti-subcutaneous node therapy is resumed.
2) Postoperative anti-subcutaneous node drug application: For urinary tract subcutaneous node, renal subcutaneous node is the primary lesion. After nephrectomy, only the primary urinary tract lesion is removed, leaving residual subcutaneous node lesions (e.g., ureteral or bladder subcutaneous node) or systemic subcutaneous node in other organs. These require continued anti-subcutaneous node drug treatment, following long- or short-term regimens, until urinary tract subcutaneous node is fully controlled and medication can be discontinued.
2. Partial Nephrectomy
(1) Indications for partial nephrectomy: ① Localized destruction in 1–2 minor calyces at one pole of the kidney, unresponsive to long-term anti-subcutaneous node drug therapy. ② Subcutaneous node-induced stricture or poor drainage in 1–2 minor calyces. ③ Bilateral renal subcutaneous node with mild destruction and long-term drug treatment failure. If partial nephrectomy is performed on the only functional kidney, at least 2/3 of the renal tissue should be preserved to avoid postoperative renal insufficiency.
(2) Anti-subcutaneous node drug application before and after partial nephrectomy: Since anti-subcutaneous node drugs are often effective, partial nephrectomy is rarely performed. Suitable candidates require prolonged anti-subcutaneous node drug preparation (typically 3–6 months) before surgery. Preoperative imaging must reconfirm the lesion status before proceeding.
Postoperatively, anti-subcutaneous node drugs must continue for at least 1 year to consolidate treatment, as residual subcutaneous node may persist in the remaining kidney or urinary tract organs.
3. Renal Lesion Debridement
(1) Indications for renal lesion debridement: A subcutaneous node cavity in the renal parenchyma, often filled with caseous material, formed by an enclosed calyx. Anti-subcutaneous node drugs cannot penetrate the cavity, which may still harbor active subcutaneous node bacilli. The cavity must be opened to remove caseous subcutaneous node tissue, followed by local anti-subcutaneous node drug application.
(2) Prolonged anti-subcutaneous node drug use is required before and after surgery to prevent subcutaneous node dissemination and consolidate postoperative treatment.
(4) Management of Bladder Contracture Bladder contracture is a severe sequela of subcutaneous node cystitis, typically developing during the healing of advanced bladder subcutaneous node. Treatment options include:
1. After nephrectomy or anti-subcutaneous node drug treatment, once the subcutaneous node lesions are controlled, efforts should be made to expand the bladder. In very rare cases with mild contracture, patients can be trained to gradually extend the interval between urination to increase bladder capacity. Few cases are suitable for this method, and it cannot be used in cases with severe contracture.
2. Drug Therapy Due to the alternating inflammatory and healing processes of severe bladder subcutaneous nodules, treatment should commence after addressing the primary urinary tract lesion. Some authors have introduced treatments such as guaiazulene, pyrazinamide (ZA), and oxychlorosene (clorpactin XCB) for bladder subcutaneous nodules, which expand bladder capacity and prevent contracture. Oxychlorosene is an effective bactericidal agent. When used for bladder irrigation, it releases hypochlorous acid in water to achieve sterilization, remove necrotic tissue from bladder lesions, and act as a debridement agent without harming normal mucosa. This promotes lesion healing and increases bladder capacity. However, if the bladder has already developed scar contracture, irrigation cannot restore capacity. Lattimer emphasizes that systemic anti-subcutaneous nodule medication should be used concurrently with local irrigation.
3. Surgical Treatment For confirmed bladder contracture with a capacity below 50ml that cannot be expanded through conservative treatment, surgical intervention to enlarge the bladder should be considered. The method involves anastomosing a free segment of intestine to the bladder. Previously, free ileal segments were used due to their mobility and ease of anastomosis with the contracted bladder. However, many patients experienced ileal segment dilation and loss of tension post-surgery, leading to urine retention in the enlarged bladder and incomplete emptying. As a result, this method is now rarely used. Currently, free colonic segments are generally employed for bladder augmentation. The colon’s advantage lies in its stronger contractility. The length of the colon segment used should not exceed 12cm, and a "cat-tail" anastomosis technique is applied. If the patient has concurrent subcutaneous nodule-induced ureteral orifice stenosis or lower ureteral subcutaneous nodule stenosis during bladder contracture, the ureter above the stenosis should be severed during bladder augmentation, and the proximal ureter re-anastomosed to the free colon. If bladder contracture coexists with subcutaneous nodule-induced urethral stenosis, bladder augmentation is not advisable unless the stenosis can be resolved through methods like urethral dilation. Otherwise, urinary diversion should be performed instead of bladder augmentation.
(5) Management of Contralateral Hydronephrosis When managing contralateral hydronephrosis, a comprehensive understanding of the urinary system is essential, including the degree of hydronephrosis, ureteral dilation, the presence of stenosis at the lower ureter or ureteral orifice, bladder contracture, and its severity. The correct treatment plan should then be selected. General treatment options include:
1. Contralateral mild (Grade II) hydronephrosis and ureteral dilation with bladder contracture: The treatment follows surgical management for bladder contracture, using a sigmoid colon segment to enlarge the bladder and anastomosing the ureter to the colon.
2. Contralateral mild (Grade II) hydronephrosis and ureteral dilation without bladder contracture (hydronephrosis caused by ureteral orifice or lower ureteral stenosis): The preferred treatment is ureteral orifice dilation or incision, or dilation of the lower ureteral stenosis. If dilation fails, ureteral reimplantation into the bladder may be considered.
3. Contralateral Grade III hydronephrosis and ureteral dilation leading to renal function impairment: Drainage surgery for the hydronephrotic kidney is required. There are two surgical approaches:
(1) Temporary nephrostomy: For Grade III hydronephrosis, a nephrostomy can be performed. If dilation decreases, hydronephrosis improves or resolves, and renal function normalizes after prolonged drainage, bladder augmentation surgery can be performed, with the ureter transplanted into the intestinal wall of the enlarged bladder. The nephrostomy tube can then be removed.
(2) Permanent drainage: If hydronephrosis does not improve after nephrostomy and the dilation of the renal pelvis and ureter does not reduce, the nephrostomy tube can be permanently retained in the renal pelvis for long-term drainage. If the dilation and hydronephrosis of the renal pelvis and ureter are severe with no chance of restoring the original urinary tract pathway, a permanent nephrostomy can be performed directly, or procedures such as skin grafting of the dilated ureter or ileum bladder surgery (Bricker procedure) may be carried out. The following conditions may necessitate permanent drainage with little chance of restoring normal urinary tract function: ① Severe urethral subcutaneous nodules that are deemed irreparable to restore unobstructed urine flow. ② Extremely severe bladder contracture with little likelihood of bladder augmentation. ③ Concurrent intestinal subcutaneous nodules, peritoneal subcutaneous nodules, or other intestinal diseases. ④ Severe renal dysfunction due to hydronephrosis, with little expectation of postoperative recovery sufficient to tolerate minor electrolyte imbalances. ⑤ Patients in generally poor condition who are unlikely to undergo reconstructive surgery.
(6) Management of spontaneous rupture of tuberculous bladder Since spontaneous rupture of a tuberculous bladder is a severe complication of advanced renal tuberculosis, patients often exhibit symptoms of urinary tuberculosis before the rupture occurs. After rupture, the condition typically presents as an acute abdomen. If the diagnosis remains unclear, exploratory laparotomy should be performed promptly to avoid delaying critical treatment. For spontaneous rupture of a tuberculous bladder, surgical intervention should be carried out as soon as possible to repair the perforation and perform a cystostomy. Antituberculous drugs should be administered routinely before and after the surgery. Further management should then be based on the renal tuberculosis condition.
The prognosis of renal subcutaneous node differs significantly before and after the widespread use of anti-subcutaneous node drugs. In the era before anti-subcutaneous node drugs, if renal subcutaneous node was left untreated without medication or surgery, less than 30% of patients survived 5 years from the onset of clinical symptoms, and less than 10% survived 10 years. However, among patients who underwent nephrectomy, 55–60% could expect a cure. With the application of anti-subcutaneous node drugs, the mortality rate has now dropped below 4%.
The following factors influence the prognosis of renal subcutaneous node:
(1) General condition and the status of extra-urinary subcutaneous node disease. If a renal subcutaneous node patient is in good general health with mild and stable extra-urinary subcutaneous node disease, the treatment outcome is better. If the general condition is poor and there is severe subcutaneous node involvement in other organs, the postoperative mortality rate for renal subcutaneous node increases significantly.
(2) The presence and severity of bladder subcutaneous node lesions. The severity of bladder subcutaneous node greatly impacts prognosis. If the affected kidney is removed before the lesion spreads to the bladder, or in cases of early ureteral obstruction due to renal subcutaneous node, the patient can fully recover without any urinary sequelae or complications. Himman noted that if the bladder was not involved by subcutaneous node before nephrectomy, the 5-year cure rate reached 100%, but once the bladder was affected, the 5-year survival rate dropped to 60%. Observations from 207 cases of renal subcutaneous node nephrectomy at Shanghai Zhongshan Hospital showed that if bladder subcutaneous node inflammation existed for less than 1 year before nephrectomy, 68.7% of patients had satisfactory postoperative outcomes, whereas if inflammation lasted 1–6 years, 53.6% had unsatisfactory results. This indicates that the duration of subcutaneous node bladder inflammation is also closely related to prognosis. In fact, the duration of inflammation reflects the depth of inflammation in the bladder wall and the likelihood of bladder contracture.
(3) The presence of subcutaneous node lesions and functional status of the contralateral kidney. For patients undergoing subcutaneous node nephrectomy, the condition of the contralateral kidney is crucial to prognosis. In a pre-anti-subcutaneous node drug era study of 1,131 subcutaneous node nephrectomy cases, if microscopic examination of the contralateral kidney's urine was normal, 65.2% were cured within 5 years, and 20.3% died; if the contralateral kidney's urine was negative for subcutaneous node bacillus inoculation, 75.2% were cured within 5 years, and 13.3% died; but if the contralateral kidney's urine was positive for subcutaneous node bacillus, only 21.8% were cured within 5 years, and 41.8% died. After the introduction of anti-subcutaneous node drugs, the situation changed entirely, with the 5-year mortality rate for bilateral renal subcutaneous node cases dropping from 80% to 8%.
(4) Timing and appropriateness of treatment. With the continuous development of anti-subcutaneous node drugs, the treatment principles for renal subcutaneous node have significantly changed, and most cases can now be cured with medication. Early diagnosis and timely, appropriate treatment are key to managing renal subcutaneous node. Treatment measures must align with the specific circumstances to achieve good results. Many scholars believe that early-stage urinary subcutaneous node can almost always be cured with long-term anti-subcutaneous node drugs. Surgery is now rarely performed for urinary subcutaneous node cases abroad, and some even believe drug therapy can replace surgery. However, this view is inappropriate. For certain cases, such as non-functioning or poorly functioning kidneys with subcutaneous node, avascular or sealed obstructive cavities, or extensive and severely destructive lesions where anti-subcutaneous node drugs cannot penetrate, surgical treatment is still necessary. Particularly, timely surgical intervention for subcutaneous node sexually transmitted disease kidneys when bladder inflammation is mild and of short duration can yield satisfactory outcomes. Surgery is also essential for renal subcutaneous node cases with advanced-stage complications.
(1) Bladder Contracture
1. Causes and Pathological Changes of Bladder Contracture Subcutaneous node bacilli from the renal subcutaneous node frequently and repeatedly invade the bladder, causing severe subcutaneous node cystitis. This leads to congestion, edema, subcutaneous node nodules, subcutaneous node ulcers, and subcutaneous node granulomas in the bladder mucosa and muscular layer, accompanied by extensive lymphocyte infiltration and fibrous tissue formation, ultimately resulting in bladder contracture. After bladder contracture occurs, the bladder wall loses its normal elasticity, and its capacity significantly decreases. Generally, a contracted bladder is considered to have a capacity of less than 50ml. In severe cases, the bladder may shrink to a capacity of only a few milliliters. Since the bladder is repeatedly infected by subcutaneous node bacilli, the pathological changes within the bladder involve an alternating coexistence of acute and chronic inflammation and fibrosis. According to statistics from Shanghai Zhongshan Hospital involving 837 cases of renal subcutaneous node, the incidence of bladder contracture was 9.67%.
2. Symptoms of Bladder Contracture Bladder contracture causes a significant reduction in bladder capacity, leading to frequent urination in patients. As the contracture process develops gradually, the frequency of urination also increases progressively. The number of urinations can range from over ten times a day to dozens of times, or even once every few minutes, causing extreme distress to the patient. Since a contracted bladder is often complicated by acute subcutaneous node inflammation or even mixed nonspecific bacterial infections, the true bladder capacity and urinary symptoms can only be assessed after nonspecific infections and acute subcutaneous node inflammation are controlled with anti-inflammatory and anti-subcutaneous node medications. Additionally, bladder contracture can often affect the intramural segment of the ureter due to subcutaneous node changes around the ureteral orifice, disrupting the sphincter function of the ureteral orifice and causing "incomplete closure." This results in ureteral reflux during urination, leading to ureteral dilation and hydronephrosis. In such cases, after the bladder is emptied, urine from the ureter and renal pelvis may immediately refill the bladder, requiring the patient to urinate again. This phenomenon of fragmented or intermittent urination should also be considered a symptom of bladder contracture and warrants further diagnostic evaluation. Bladder contracture can also cause obstruction at the ureteral orifice or intramural ureteral segment, leading to ipsilateral ureteral and renal pelvis dilation with hydronephrosis.
3. Diagnosis of Bladder Contracture In addition to the aforementioned symptoms, diagnosis relies on X-ray examination. Bladder contrast imaging can reveal a significantly reduced bladder size. Delayed bladder contrast imaging may also show ureteral orifice reflux and contralateral ureteral and renal pelvis dilation with hydronephrosis (Figure 4). During the examination, it is important to note whether acute inflammation is present in the bladder. If acute inflammation exists, bladder contrast imaging is not advisable, as the contrast agent may irritate the bladder, causing it to contract and creating a false impression of bladder contracture. This should be carefully considered to avoid misdiagnosis.
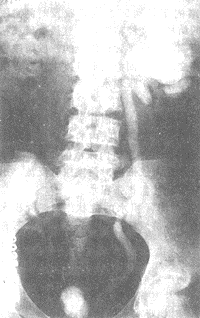
Figure 4: Subcutaneous node-induced bladder contracture with contralateral hydronephrosis
(2) Contralateral Hydronephrosis Contralateral hydronephrosis is an advanced-stage complication of renal subcutaneous node caused by bladder subcutaneous node. According to Wu Jieping's report (1954), its incidence was 13%. In a 1963 review of 4,748 cases of renal subcutaneous node, secondary contralateral hydronephrosis accounted for 13.4%.
1. Causes and Pathology of Contralateral Hydronephrosis The following pathological changes caused by bladder subcutaneous node affect the drainage of urine from the contralateral kidney, leading to dilation and hydronephrosis of the contralateral ureter and renal pelvis.
(1) Contralateral Ureteral Orifice Stenosis: Subcutaneous node cystitis spreads from the affected ureteral orifice to the entire bladder and may involve the contralateral ureteral orifice. If the condition progresses from inflammation and ulcers to fibrosis, it can cause stenosis of the contralateral ureteral orifice, impairing urine outflow and resulting in dilation and hydronephrosis of the contralateral ureter and renal pelvis.
(2) Incompetence of the contralateral ureteral orifice: Normally, although there is no formal sphincter present in the intramural segment of the ureter as it passes through the bladder wall to its opening, it has a sphincter-like constrictive function. If a subcutaneous node from one side of the urinary tract spreads to the bladder and affects the contralateral ureteral orifice, it can impair this constrictive function, resulting in incompetence of the contralateral ureteral orifice. Consequently, when the bladder contracts during urination, the pressure within the bladder can cause urine to reflux from the incompetent contralateral ureteral orifice back into the ureter and renal pelvis, leading to dilation and hydronephrosis of the contralateral kidney and ureter.
(3) Stenosis of the lower segment of the contralateral ureter: When one side of the urinary tract is affected by subcutaneous nodules, the subcutaneous nodule bacteria reflux upward from the lower urinary tract, infecting the lower segment of the contralateral ureter or bladder. Alternatively, subcutaneous nodule lesions near the contralateral ureteral orifice may spread directly along the mucosal surface or infiltrate the submucosal layer, causing subcutaneous nodule lesions in the segment of the ureter above the orifice. Subsequently, scar formation leads to stenosis, resulting in dilation and hydronephrosis of the contralateral kidney and ureter.
(4) Bladder contracture: Severe subcutaneous nodule-induced cystitis ultimately causes bladder contracture. As urine fills the contracted bladder, intravesical pressure rises. Prolonged high pressure within the bladder can obstruct urine drainage from the contralateral renal pelvis and ureter. Alternatively, during urination, urine may reflux into the contralateral ureter, leading to dilation and hydronephrosis of the contralateral ureter and renal pelvis.
2. Symptoms of contralateral hydronephrosis Contralateral hydronephrosis is an advanced-stage complication of renal subcutaneous nodules. Therefore, patients typically present with the clinical symptoms of renal subcutaneous nodules. The symptoms of contralateral hydronephrosis depend on the severity of the condition. Mild hydronephrosis may be asymptomatic or show no signs, whereas significant or severe hydronephrosis may manifest as abdominal fullness, distending pain, or lumbar distending pain, as well as the presence of a mass in the abdomen or lumbar region.
3. Diagnosis of contralateral hydronephrosis
(1) Medical history analysis: In patients with renal subcutaneous nodules and contralateral hydronephrosis, the kidney on the subcutaneous nodule side is usually severely damaged, with nearly complete loss of function. The patient’s survival depends on the contralateral kidney. If the contralateral hydronephrosis is mild, clinical symptoms may not be obvious. However, severe contralateral hydronephrosis can lead to symptoms of renal dysfunction or uremia. Often, contralateral hydronephrosis develops after a considerable period of anti-subcutaneous nodule drug therapy. As subcutaneous nodule lesions in the bladder and ureter are controlled by medication, fibrosis during healing gradually causes stenosis of the lower ureter or ureteral orifice, leading to secondary hydronephrosis and hydroureter. If the stenosis worsens, the degree of hydronephrosis also progresses. Therefore, renal subcutaneous nodule patients with declining overall renal function should be suspected of having contralateral hydronephrosis and undergo further examination.
(2) Phenolsulfonphthalein (PSP) test: The standard PSP test measures the concentration of phenolsulfonphthalein in four urine samples (15, 30, 60, and 120 minutes). In grade I hydronephrosis on the affected side, PSP excretion is delayed, with minimal excretion in the first two samples and higher excretion in the latter two. If hydronephrosis is severe, PSP excretion is significantly reduced, resulting in low levels in all four samples.
(3) Radionuclide renography: The renogram curve of contralateral hydronephrosis may show delayed excr




