| disease | Esophageal Cancer (Surgery) |
| alias | Malignant Neoplasm of Oesophagus |
Esophageal tumors are mostly malignant, with esophageal cancer being the most common among malignant cases. China has one of the highest incidence rates of esophageal cancer in the world, with the highest mortality rate globally.
bubble_chart Etiology
Esophageal cancer has the characteristic of high-incidence areas, indicating that these regions possess conditions conducive to its occurrence, such as the presence of strong carcinogens and tumor promoters, a lack of certain anti-cancer factors, and genetic susceptibility. However, research findings vary significantly across countries and regions, reflecting the diverse etiological factors of esophageal cancer. Western scholars often attribute smoking and alcohol consumption as the primary causes. In Lin County, China, a high-incidence area, poverty meant that residents only began consuming alcohol in the past decade or two. Before that, basic sustenance was scarce, let alone excessive drinking.
(1) Nitrosamine Compounds
It is well-known that nitrosamines can induce upper digestive tract cancer in animals. In the environment of Lin County, a high-incidence area, seven volatile nitrosamines were detected, with high positive rates for dimethylnitrosamine (64%), dipropylnitrosamine (30%), and diethylnitrosamine (24%). Non-volatile nitrosamines such as nitrososarcosine were found in cornmeal, and nitrosoproline in pickled radish. Nitrites and nitrates were also detected at high levels in contaminated foods in Lin County, showing a positive correlation with the nitrosamine compounds identified. Secondary and tertiary amines are widely distributed in food and the environment. Although environmental nitrosamine levels are minimal, under acidic conditions in the stomach, amines and nitrites readily combine to form nitrosamines, which may be the primary source. Recent studies used methylbenzylnitrosamine (NMBzA), discovered in the Lin County environment, to co-culture with human fetal esophageal epithelial cells for three weeks. The epithelial cells were then transplanted onto the mesentery of BALB/C nude mice, while the mice continued to be fed NMBzA. The result was the development of squamous cell carcinoma on the mesentery, with no tumors in the esophagus. No tumors were observed in the control group. DNA from the induced tumors was subjected to nucleic acid hybridization with the human-specific repetitive sequence—Alu sequence. The presence of Alu sequences in the tumors confirmed their human tissue origin. This experiment provided the first direct evidence that nitrosamines can induce human esophageal squamous cell carcinoma, supporting the nitrosamine etiology of esophageal cancer in Lin County.
(2) Nutritional Factors and Trace ElementsWhether domestically or internationally, high-incidence areas of esophageal cancer are typically impoverished and underdeveloped, with harsh natural conditions, scarce water resources, limited agricultural output, and inadequate food supplies. Diets in these areas lack animal protein, fats, fresh vegetables, and fruits. Surveys confirmed that farmers in Lin County have insufficient riboflavin intake, as well as low levels of vitamins A and C. Vitamin C can block the synthesis of carcinogenic N-nitroso compounds, while riboflavin deficiency significantly increases the incidence of esophageal tumors induced by methylbenzylnitrosamine in rats and shortens the latency period.
Surveys also found that drinking water in high-incidence areas has relatively low levels of trace elements such as molybdenum, zinc, copper, and magnesium. Trace elements are essential components of certain oxidases and nitrate reductases. Molybdenum deficiency can lead to nitrate accumulation in plants. It has been confirmed that applying molybdenum fertilizer can increase molybdenum content in food and reduce nitrite levels.
(3) Pickled Vegetables and Mold
High-incidence areas like Lin County, Yangquan in Shanxi, Yanting in Sichuan, and Yangzhong in Jiangsu commonly consume pickled vegetables. Experiments have demonstrated the presence of carcinogens and tumor promoters in pickled vegetables from Lin County. Studies also found that 55% of pickled vegetables contain a nitroso compound called Roussin red methyl ester, which can transform C3H/10T1/2 cells initiated by 3-methylcholanthrene, suggesting it may act as a tumor promoter in pickled vegetables.
(4) Research on Esophageal Cancer OncogenesEsophageal Cancer Oncogenes: Analysis of DNA from esophageal cancer tissues and adjacent epithelial tissues revealed that most cases involve amplification and enhanced expression of EGFr and c-myc genes. Other genes with amplified expression include int-2, Cyclin D, and HER-1. The overexpression and amplification of these genes may be closely related to the development of human esophageal cancer.
In esophageal cancer tissues, the tumor suppressor genes: approximately one-third of esophageal cancers and adjacent cancerous tissues exhibit structural abnormalities in the retinoblastoma susceptibility gene Rb, with complete or partial loss of fragments. Nitrosamines play a key role in this loss. Another tumor suppressor gene, P53, shows structural abnormalities in 11–14% of esophageal cancer tissues and adjacent epithelial tissues. In the DNA of human fetal esophageal epithelial cells induced to become cancerous by N-methylbenzylnitrosamine (NMBzA), partial loss of the P53 gene was observed. Additionally, high expression of P53 was found in both esophageal cancer tissues and their corresponding adjacent epithelial tissues.
In the high-incidence area of esophagus cancer, Linxian, the transforming genes in esophagus cancer tissues were studied. DNA from 8 cases of esophagus cancer tissues was transfected into NIH/3T3 cells using the DNA-Ca3(PO4)2 coprecipitation-mediated transfection technique. It was found that 3 cases exhibited transforming activity and underwent a second round of transformation. The uncloned NIH/3T3 cells differed in morphological characteristics from spontaneously transformed cells. The first-round transfection rate was low, at 0.025–0.05 foci/μg DNA. The second-round transfection frequency significantly increased to 0.30 foci/μg DNA. Both first- and second-round transformed cells were capable of forming colonies in 0.33% soft agar. NIH/3T3
cells from normal controls showed no anchorage-independent growth. Through dot blot and Southern hybridization techniques, the human highly repetitive Alu sequence was detected in first- and second-round transformed cells, demonstrating that the malignant transformation of NIH/3T3 cells was induced by DNA from esophagus cancer tissues. This proves the presence of a dominant gene—the transforming gene—in esophagus cancer tissues that can induce in vitro transformation of NIH/3T3 cells.In vitro experiments with human esophageal epithelium and in vivo studies in rhesus monkeys demonstrated that the chemical carcinogen N-methyl-N-benzylnitrosamine (NMBzA) can activate cellular proto-oncogenes during the initiation stage of carcinogenesis, suggesting it may be a cause rather than a consequence of cancer.
The development of esophagus cancer is the result of the action of multiple oncogenes.
bubble_chart Pathological Changes
In esophageal cancer, squamous cell carcinoma accounts for 90–95%, adenocarcinoma originating from glands accounts for 5–7%, and rare cases include adenoacanthoma (a combination of squamous cell carcinoma and adenocarcinoma), fleshy tumor, and undifferentiated carcinoma. Clinical and pathological statistics from domestic and international sources indicate that the middle segment is the most common site, accounting for approximately 50%, followed by the lower segment at 33.2–43.3%, and the upper segment being the least common at 5.8–15.3%.
Gross specimens of esophageal cancer are classified into two major categories: early-stage and middle-to-advanced-stage. Early-stage refers to carcinoma in situ and early invasive carcinoma. According to literature reports, macroscopic findings are further divided into: ① Occult type: accounts for 7.3–11.8%, with grade I localized congestion and pink discoloration of the mucosal membrane, all of which are carcinoma in situ under microscopy. ② Erosive type: accounts for 33–51.2%, with grade I erosion and congestion of the mucosal membrane, irregular morphology mixed with normal pink-white mucosa in a map-like pattern, and microscopy showing equal proportions of carcinoma in situ and early invasive carcinoma. ③ Patch type: accounts for 24.4–51.3%, with slightly elevated, swollen, and thickened mucosal membrane, rough surface, pale-white appearance after fixation, and thickened or interrupted longitudinal or transverse mucosal folds. Histologically, carcinoma in situ accounts for 1/3, and early invasive carcinoma accounts for 2/3. ④ Papillary type: accounts for 8.0–12.6%, with lesions protruding into the lumen like papillae or polyps, mostly smooth mucosal surface, occasionally grade I erosion, and microscopy showing mostly early invasive carcinoma.
Middle-to-advanced-stage esophageal cancer is grossly classified into: ① Medullary type (massive type): accounts for 56.8%, with a large tumor volume, asymmetrical thickening of the affected esophageal wall involving most or the entire circumference, slope-like elevation at both ends, and ulceration. ② Fungating type: accounts for 18.5%, with a mushroom-like tumor protruding into the lumen, clear and everted borders, and a large, shallow ulcer, involving part or most of the esophageal circumference. ③ Ulcerative type: accounts for 13.3%, with thin cancerous tissue involving part of the esophageal circumference, presenting as a deep ulcer prone to perforation. ④ Constrictive type: accounts for 8.5%, with a short, ring-like stricture usually involving the entire circumference, length not exceeding 5 cm, surface erosion, mostly without ulceration, and marked dilation above the stricture. ⑤ Intraluminal type: accounts for 3%, with a round or oval tumor protruding into the esophageal lumen, connected to the esophageal wall by a stalk of varying thickness, and surface erosion or small shallow ulcers. Surgical treatment yields better outcomes for the fungating type, intermediate results for the ulcerative and medullary types, poorer results for the constrictive type, and although the resection rate is high for the intraluminal type, long-term outcomes are poor.
Staging and segmentation of esophageal cancer
At the 1976 National Esophageal Cancer Treatment Experience Conference held in Yangquan City, Shanxi, Chinese scholars proposed a staging system based on lesion length, depth, lymph node metastasis, and organ metastasis (Table 1).
Table 1 Clinical and pathological staging of esophageal cancer (1976 Yangquan Conference)
| Stage | Lesion length | Lesion extent | Metastasis status | |
| Early stage | 0 | Not specified | Limited to mucosa (carcinoma in situ) | (-) |
| Ⅰ | <3 cm | Invades submucosa (early invasion) | (-) | |
| Intermediate stage [second stage] | Ⅱ | 3~5cm | Invasion of partial muscle layer | (-) |
| III | >5cm | Penetration of muscle layer or external invasion | Regional lymph nodes (+) | |
| IV | >5cm | Significant external invasion | Distant lymph nodes (+) or organ metastasis |
Through practice, scholars both domestically and internationally have found that among the above four indicators, the most significant factors determining prognosis are the extent of the lesion, lymph node metastasis, and distant metastasis, while the length of the lesion is less closely related to prognosis. In 1987, the International Union Against Cancer (UICC) proposed the TNM staging system for esophageal cancer (Table 2).
Table 2 Comparison between UICC esophageal cancer staging (1987) and Chinese staging
| UICC Stage | Tumor T | Lymph Node N | Metastasis M | Chinese Staging | |
| Pathology | Clinical | ||||
| 0 | Tis | N0 | M0 | 0 | |
| I | T1 | N0 | M0 | I | <3cm |
| IIA | T2 | N0 | M0 | II | 3-5cm |
| IIB | T3 | N0 | M0 | III | >5cm |
| T1 | N1 | M0 | |||
| T2 | N1 | M0 | |||
| III | T3 | N1 | M0 | ||
| IV | T4 | Any N | M0 | IV | |
| Any T | Any N | M1 | M1 | ||
In the table, Tis represents carcinoma in situ, T1 indicates tumor invasion into the submucosa, T2 indicates tumor invasion into the muscular layer, T3 indicates tumor penetration through the muscular layer reaching the fibrous membrane. The limitation of this staging method lies in the insufficient detail in classifying lymph node metastasis, failing to distinguish between first and second stations, which requires future improvement.
Esophageal segmentation method: Since 1940, when Wu Yingkai successfully performed the first resection of esophageal cancer in China, the following segmentation method has been adopted: the upper segment extends from the esophageal entrance (approximately at the level of the 6th cervical vertebra) to the upper edge of the aortic arch, further divided into the cervical segment from the esophageal entrance to the sternal notch and the upper thoracic segment from the sternal notch to the upper edge of the aortic arch (upper edge of T4); the middle segment extends from the upper edge of the aortic arch to the lower edge of the inferior pulmonary vein (lower edge of T6), and the lower segment extends from the lower edge of the inferior pulmonary vein to the cardia (including the subdiaphragmatic abdominal segment of the esophagus). The drawbacks of this segmentation method are: ① The aortic arch as a landmark is not constant, as it elongates and shifts upward with age; ② The lower thoracic segment is relatively short, while the middle thoracic segment is relatively long, resulting in unequal division; ③ The inferior pulmonary vein is sometimes indistinct and difficult to identify, and the middle segment includes both the upper and lower parts of the tracheal bifurcation, referred to as the high and low middle segments, but their resection rates and prognoses differ significantly. The anatomical segmentation of the esophagus traditionally used in China is shown in Figure 2.
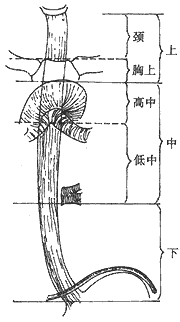
Figure 2 Traditional Anatomical Segmentation Standard in China
The segmentation method proposed by the International Union Against Cancer (UICC) is shown in Figure 3.
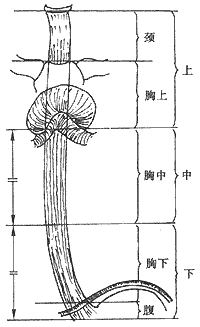
Figure 3 New UICC Esophageal Segmentation Standard
UICC esophageal segmentation standard: ① The cervical segment extends from the cricoid cartilage to the thoracic inlet (sternal notch) (approximately 18 cm from the upper incisors). The intrathoracic segment is divided into three parts. ② The upper thoracic segment extends from the thoracic inlet to the tracheal bifurcation (approximately 24 cm from the upper incisors). ③ The middle thoracic segment is the upper half of the length from the tracheal bifurcation to the esophagogastric junction (lower boundary approximately 32 cm from the upper incisors). ④ The lower thoracic segment is the lower half of the above division (lower boundary approximately 40 cm from the upper incisors). The new segmentation method has the advantages of clear landmarks, uniform segment lengths, and significant correlation between intrathoracic segments and prognosis, and should be widely adopted.
bubble_chart Clinical Manifestations
The most characteristic early symptoms include: ① A burning sensation or slight stabbing pain behind the sternum during swallowing, particularly noticeable when consuming rough, overheated, or irritating foods. This pain can be temporarily relieved by dysmenorrheal medication but soon recurs, and such a course may last for months or even 2–3 years. Some perceptive patients can precisely pinpoint the location of the pain. ② A sensation of slow food passage, retention, or a foreign object adhering to the esophageal wall. ③ Grade I choking sensation, referred to as "pressure on the breath" by farmers in Lin County, meaning the feeling of gas obstructing smooth swallowing. This symptom fluctuates in severity until it becomes persistent. ④ Less common symptoms include a dull sensation behind the sternum, dryness, and tightness in the throat.
In advanced-stage esophageal cancer, the typical symptom is progressive dysphagia. As the tumor destroys the muscular wall, invades the entire circumference, and obstructs the lumen, the affected segment of the esophagus loses elasticity and forms an irregularly narrowed passage. The choking symptom worsens progressively, starting with the inability to consume solid foods and gradually advancing to difficulty swallowing semi-liquid or liquid diets. Although there may be brief, unexplained improvements in swallowing, the overall trend is progressive worsening of choking. Accompanying the choking symptom is the vomiting of mucus, which is actually saliva and esophageal secretions refluxing due to obstruction preventing entry into the stomach. If aspirated into the respiratory tract, it can lead to choking coughs and pneumonia. Difficulty eating places the patient in a chronic state of prolonged hunger, inevitably accompanied by dehydration and malnutrition, with significant weight loss being a poor prognostic sign. If the affected esophageal segment has ulcers, inflammation, or tumor invasion, persistent dull pain in the chest and back may occur. Severe pain accompanied by fever should raise suspicion of tumor perforation, either imminent or already occurred. Generally, without proper treatment, the patient's condition rapidly deteriorates to a state of cachexia. When the tumor invades adjacent organs and perforation occurs, complications such as esophageal-bronchial fistula, mediastinal abscess, pneumonia, lung abscess, or major stirred pulse perforation with massive hemorrhage may lead to death. The natural survival period for advanced-stage patients is typically around 8 months. Other advanced-stage symptoms include hoarseness due to compression of the recurrent laryngeal nerve, enlargement of superficially metastatic lymph nodes, pain from bone metastases, and hepatomegaly and jaundice from liver metastases. Patients exhibiting these symptoms have already lost the opportunity for curative treatment.
The most commonly used methods include three types: X-ray barium meal contrast, exfoliative cytology, and fiber-optic endoscopy. With technological advancements, chest CT scans and esophageal endoscopic ultrasonography have also been applied clinically in well-equipped facilities. In clinical practice, the examination should proceed from simple to complex, with the first three tests being indispensable, especially fiber-optic endoscopy, which is superior to X-ray in terms of localization, length measurement, detection of secondary cancers, and exclusion of benign stenosis.
X-ray barium meal contrast often fails to reveal lesions in early-stage esophageal cancer. If the examiner follows routine procedures—using overly thick or thin barium, having the patient drink large gulps of barium, or simply observing in frontal and lateral views—it can lead to misdiagnosis. The barium meal must be properly prepared, and the patient should take small sips repeatedly while being observed meticulously from multiple angles. Early X-ray signs include: ① thickening, tortuosity, or dotted interruption of the mucosal folds, or a fuzzy esophageal margin; ② small filling defects, either flat or polypoid, with a minimum diameter of about 0.5 cm; ③ small ulcer niches, with diameters ranging from 0.2 to 0.4 cm; and ④ localized rigidity of the esophageal wall or barium retention. Due to the subtlety of the lesions, the positive detection rate of X-ray barium meal examination in early-stage cases is only about 70%. In advanced-stage cases, the signs are more definitive, often showing luminal narrowing, filling defects, loss of peristalsis, mucosal disarray, ulcer niches, and soft tissue shadows around the affected esophageal segment. Intraluminal-type X-ray barium meal contrast reveals large filling defects with widening of the affected segment.
Esophageal exfoliative cytology is a simple method with minimal patient discomfort and a low false-positive rate. It has proven to be the most practical approach for large-scale screening in high-risk areas, with an overall positive detection rate of around 90% (94.2% for esophageal cancer and 82.1% for cardia cancer), a false-positive rate of less than 1%, and a false-negative rate of approximately 10%. Some authors perform segmental multiple balloon cytology tests to localize the lesion. For example, if the test is positive above 25 cm from the incisors, a major esophageal resection with cervical reconstruction is indicated; if positive between 25–35 cm, a major resection with reconstruction above the aortic arch is performed; and if positive below 35 cm, resection and reconstruction below the aortic arch may suffice. However, this method has some margin of error, especially when the lesion is near the boundaries of these segments. Well-equipped hospitals should still rely on endoscopic localization. Notably, the positive rate of exfoliative cytology decreases in advanced-stage cases due to severe stenosis preventing the balloon from passing through the tumor segment. Contraindications for exfoliative cytology include hypertension, esophageal varices, and severe cardiac or pulmonary diseases.
The third commonly used diagnostic method is endoscopy. Since the gradual replacement of metal rigid tubes with fiber-optic scopes in the 1970s, due to their flexibility, allowing patients to assume free positions, excellent illumination, and a wide field of view (with slight magnification), the safety and accuracy of examinations have been significantly improved. The indications for fiber-optic esophagoscopy include: ① Early-stage patients with no symptoms or mild symptoms. When X-rays show no definitive findings but exfoliative cytology is positive. ② X-ray findings that are difficult to differentiate from benign sexually transmitted disease lesions, such as symmetrical, smooth narrowing resembling benign scar stenosis or submucosal wall lesions like leiomyomas. ③ Confirmed benign esophageal sexually transmitted disease lesions, such as diverticula or achalasia, when symptoms significantly worsen. ④ Follow-up of patients who have undergone various treatments to observe efficacy. Fiber-optic endoscopy also has contraindications, including: ① Cachexia; ② Severe cardiovascular disease; ③ Acute respiratory infections. Contraindications for metal tubes in the past, such as kyphosis deformity or esophageal varices, are no longer considered in fiber-optic endoscopy. In early-stage esophagus cancer, the detection rate of fiber-optic scopes can reach 85.2%. Early endoscopic manifestations include: ① Localized erosion, the most common at 53%; ② Local mucosal congestion with unclear boundaries at 38.5%; ③ Rough small granules at 27.4%. Other less common findings include small masses at 9.4%, small ulcers at 6.8%, and small patches at 6.8%. To improve the detection rate of fiber-optic endoscopy, vital staining methods (such as toluidine blue or Lugol's iodine solution) can be used during the examination. The endoscopic findings of mid-to-advanced-stage esophagus cancer are more definitive and easily recognizable, presenting as nodular or cauliflower-like masses, esophageal mucosal congestion, edema, or pallor and stiffness, prone to bleeding upon touch, and may also show ulcers or lumen narrowing. If the esophageal lesion is located in the upper thoracic or cervical segment, fiber-optic bronchoscopy should be performed simultaneously during esophagoscopy to rule out tracheal or bronchial compression or invasion.
The role of chest CT in the diagnosis and treatment of esophageal cancer varies among different evaluations. Some believe that CT is helpful for staging, assessing resectability, and estimating prognosis. However, others argue that this examination is of little value, with some authors reporting that the accuracy of CT staging is only 60%. The significant CT-positive findings are briefly summarized as follows: ① Possible invasion of the trachea or bronchi. CT may show displacement or compression of the trachea or bronchi, with the posterior wall bulging into the lumen and the disappearance of the fat layer between the esophagus and these structures. ② Possible invasion of the pericardium or aorta. The fat plane between the pericardium or aorta and the affected segment of the esophagus disappears, while the fat layers above and below the tumor remain intact. Alternatively, the angle of contact between the esophageal lesion and the aorta is equal to or greater than 90 degrees. ③ Metastasis to mediastinal or abdominal lymph nodes, with lymph nodes larger than 1 cm in diameter. ④ Liver metastasis, manifested as hypodense areas in the liver. When CT assesses invasion of mediastinal organs, the sensitivity for aortic invasion is 88%, for tracheobronchial invasion is 98%, and for pericardial invasion is 100%. For lymph node metastasis, the sensitivity of CT for detecting periesophageal lymph node metastasis is 60%, slightly higher for abdominal lymph node metastasis at 76%, with a specificity of 93%. The sensitivity of CT for detecting liver metastasis is 78%, with a specificity of 100%. Objectively, CT findings cannot distinguish whether normal-sized lymph nodes are metastatic, nor can they confirm whether enlarged lymph nodes are due to inflammation or metastasis. Furthermore, CT cannot detect metastatic lymph nodes smaller than 1 cm in diameter. As mentioned, the accuracy of assessing organ invasion is limited. Therefore, surgical opportunities should not be abandoned based solely on "positive findings" from CT.
Endoscopic Ultrasound of the Esophagus
In recent years, endoscopic ultrasound (EUS) has gradually been applied in clinical practice. However, due to the high cost of equipment, it is unlikely to be widely adopted in the foreseeable future. The endoscopic ultrasound system operates through a water-filled balloon. Normally, the first layer (mucosa) is echogenic, the second layer (muscularis propria) appears as a dark area, and the third layer (submucosa) is echogenic. The advantages of this new examination method include: ① Precise measurement of the depth of tumor infiltration within the esophageal wall, with an accuracy of up to 90%. ② Detection of abnormally enlarged lymph nodes outside the wall, including those distant from the lesion, with a detection rate of 70%. ③ Quick and easy differentiation between lesions located inside the esophagus or outside the wall. However, there are limitations: ① The detection range is limited to within 4 cm from the center of the instrument's main shaft, meaning only areas close to the esophagus or stomach can be examined. ② Structures that interfere with ultrasound cannot be present in between. ③ When the affected segment is severely narrowed and the probe cannot pass, lymph nodes adjacent to the esophagus below the lesion cannot be detected.
Abdominal ultrasound can detect retroperitoneal lymph node metastasis, liver metastasis, etc., aiding in staging and determining surgical indications. Especially for patients with gastric cardia cancer, when enlarged retroperitoneal or posterior gastric lymph nodes are detected, exploration often reveals that the actual size of the lymph nodes is much larger than what ultrasound suggests, indicating that the disease has reached a stage where radical resection is no longer feasible.
bubble_chart Treatment Measures
In our country, esophageal cancer is a common malignancy, with surgery and radiotherapy being the two treatment methods currently recognized as having definite efficacy. Through extensive clinical practice, both thoracic surgery and radiation oncology departments have accumulated rich experience, with treatment outcomes ranking among the forefront internationally.
One of the trends in the surgical treatment of esophageal cancer is the gradual expansion of surgical indications. In the 1960s, lesions located in the cervical and upper thoracic segments were typically treated with radiotherapy rather than surgery, with surgical cases accounting for only 5% of the total. This bias has since changed. Starting from the 1970s, an increasing number of esophageal cancers in these segments have been treated surgically with satisfactory outcomes. The proportion of surgical cases has risen to 15–20% of the total and is expected to continue increasing. Another manifestation of expanded indications is the growing number of esophageal cancer patients with various internal diseases undergoing surgical treatment.
The main issue currently is the limited number of cases that seek medical attention at an early stage. The solution lies in continuing to vigorously promote cancer prevention awareness and improving vigilance toward early symptoms among both patients and doctors.
(1) Indications for Surgical Treatment of Esophageal Cancer
1. UICC stages 0, I, IIA, IIB, and III with T 3 N 1 M 0.
2. Cases where radiotherapy has failed to control the disease or where recurrence has occurred, without obvious local invasion or distant metastasis.
3. Generally, patients should not exceed 70 years of age, though a few elderly patients nearing 80 with a younger physiological age may also be cautiously considered. The length of the lesion is not strongly correlated with treatment prognosis and is only a reference indicator in decision-making.
Contraindications for surgery include: ① Cachexia; ② Late-stage III (T 4 any M 0) and stage IV according to UICC staging; ③ Severe comorbidities in vital organs, such as poor lung function, heart disease with heart failure, or myocardial infarction within the past six months.
For thoracic esophageal cancer with cervical lymph node metastasis, according to the 1987 TNM staging, this is classified as distant lymph node metastasis (M 1 ). In the past, such cases were treated non-surgically. For every patient considered for surgery, the surgeon should preoperatively assess the likelihood of resection based on: ① The location of the lesion—resection rates are lowest for upper-segment lesions (66.7%–89.5%), followed by middle-segment lesions (79.1%–94.5%), and highest for lower-segment lesions (87.2%–98.4%). ② The direction of the esophageal segment with the lesion—if it deviates from the normal segment, exhibiting a twisted angle, this suggests a large tumor volume with external invasion or compression by large metastatic lymph nodes, reducing the likelihood of resection. ③ The position and depth of ulcerative niches in the lesion—if an ulcer is located on the left side of the middle esophageal segment or extends beyond the esophageal wall, it indicates tumor invasion into the mediastinum or impending perforation into the lung, bronchus, or even the aorta, making resection (especially radical resection) less likely. ④ The presence of soft tissue shadows—if large soft tissue masses are visible on plain X-rays or CT scans, compressing the trachea, bronchi, pericardium, or encircling more than a quarter of the aorta, the likelihood of resection decreases. ⑤ Pain symptoms—severe chest or back pain suggests invasion into sensitive structures like the mediastinal pleura, making resection unlikely.
(2) Other Conditions for Surgical Treatment of Esophageal Cancer
The indications for surgical treatment of esophageal cancer, in addition to the disease stage not being later than stage III and the T-stage preferably before T4, also involve consideration of three main issues. First is the patient's nutritional status. Patients with esophageal cancer, due to long-term progressive dysphagia, generally exhibit a negative metabolic balance, manifested as significant weight loss and emaciation. Moreover, due to forced dietary restrictions, they not only suffer from hypoalbuminemia but also deficiencies in other nutrients such as vitamins, electrolytes, and trace elements. These conditions adversely affect the patient's cardiovascular system, weaken their resistance to infection, and impair wound (including anastomotic) healing, necessitating proper preoperative correction. The second issue pertains to the patient's cardiopulmonary function. Patients with poor lung function have a significantly increased risk of postoperative pulmonary complications. Since esophageal cancer patients are mostly elderly individuals over 50 years old, they often suffer from chronic bronchitis, pulmonary emphysema, and other conditions that impair function. Although there are numerous pulmonary function indicators, the most clinically relevant is the forced expiratory volume in one second (FEV1). An ideal FEV1 value is above 75% of the estimated value, making such patients suitable for surgery. If the value is below 75% but above 50%, surgery should be carefully considered. If it is below 50%, surgery is generally contraindicated, though not absolutely. As for cardiac function, aside from ensuring no episodes of angina or heart failure within the past six months, simple questioning can often gauge the patient's functional reserve. For example, if the patient can walk two miles at a normal pace or climb three flights of stairs without stopping, their cardiac reserve should be sufficient to withstand the surgical burden. Radionuclide blood pool scanning should show a resting left ventricular ejection fraction of at least 40%, with an increase after exercise. If the ejection fraction is below 40% or fails to increase after exercise, further evaluation with coronary angiography or ventriculography is warranted. The final frequently encountered issue is determining the upper age limit for surgical treatment of esophageal cancer. In the past, this was often set at 70 years. However, with increasing average life expectancy, individuals over 70 are now commonplace. Nevertheless, surgical risks rise correspondingly with age, and data indicate that the mortality curve associated with surgery steepens after the age of 75. For esophageal cancer patients over 75, palliative surgery to alleviate symptoms should be prioritized over curative radical surgery, as palliative resection yields better outcomes than radiotherapy or intraluminal stenting. However, for patients over 80, resection carries excessive risk, and alternative palliative methods are more appropriate.
(3) Surgical Approaches for Esophageal Cancer
The surgical approaches for esophageal cancer include left posterolateral thoracotomy, right posterolateral thoracotomy combined with laparotomy (or freeing the stomach through the esophageal hiatus), left posterolateral thoracotomy combined with a left cervical incision (double incision), left cervical and right posterolateral thoracotomy combined with laparotomy (triple incision), non-thoracotomy cervical and abdominal double incision (esophageal inversion and stripping), and median sternotomy for the upper mediastinal approach. The choice of approach depends mainly on the surgeon's preference and the patient's condition. The main advantages of the left posterolateral approach are: ① It provides excellent exposure for mid-to-lower esophageal cancer and cardiac cancer. ② Through the left diaphragmatic incision, it is relatively easy to free and dissect the stomach, perform lymph node dissection around the gastric cardia, left gastric vessels, and esophagus, and finally resect the esophageal cancer and relocate the stomach into the thoracic cavity for esophagogastric anastomosis below or above the aortic arch, restoring the continuity of the upper digestive tract. In other words, a single left thoracotomy incision is sufficient for both partial esophagogastrectomy and esophagogastric anastomosis. ③ Since the main structures are well exposed, accidental injuries are less likely to occur, and even if they do, they are easier to repair and control bleeding. ④ When the cardiac cancer is more extensive than preoperatively estimated, requiring more radical surgery (such as total gastrectomy or partial resection of the stomach, spleen, and pancreas), extending the incision anteriorly and inferiorly into the abdomen by cutting the costal cartilage arch, extending the diaphragmatic incision, and partially incising the abdominal muscles converts it into a left thoracoabdominal combined incision. This incision provides satisfactory exposure of the upper abdomen, making it easier to free the entire stomach or colon. The limitation of the left posterolateral incision is the difficulty in dissecting lesions above the aortic arch. If the resection above the arch is incomplete, a left cervical incision should be added for resection and reconstruction in the neck. The right posterolateral thoracotomy combined with laparotomy (double incision) is rarely used in our institution, mainly because it is less convenient than the left posterolateral thoracotomy, which allows both thoracic and abdominal procedures through a single incision. The triple incision (left cervical, right posterolateral thoracotomy, and upper midline laparotomy) is suitable for upper thoracic lesions requiring cervical reconstruction. The patient is first placed in the left lateral position for right posterolateral thoracotomy to dissect and free the lesion and normal esophagus, followed by chest closure. The patient is then repositioned supine for laparotomy to free the stomach or colon, which is pulled up to the neck through the esophageal bed for digestive tract reconstruction. The right posterolateral incision facilitates mediastinal lymph node dissection better than the left posterolateral incision, improving the radicality of resection. The drawback is the need for repeated repositioning and draping, prolonging the operation time. Some surgeons recommend a triple incision of right anterolateral thoracotomy combined with right cervical and abdominal incisions, allowing single positioning and draping without repetition. However, the disadvantage is poorer exposure compared to the right posterolateral approach, requiring blind blunt dissection for a significant portion of the esophageal dissection.
The non-thoracotomy cervical and abdominal double incision is suitable for patients with poor cardiopulmonary function who cannot tolerate thoracotomy. Esophageal separation is performed downward through the cervical incision and upward through the abdominal incision via the hiatus, using fingers or instruments for blunt dissection. The advantage is faster and more stable postoperative recovery, but the drawback is that it violates basic surgical principles—there is no exposure—and does not adhere to oncologic principles, as it fails to completely resect the lesion and metastatic lymph nodes. Although proponents of this approach report a 5-year survival rate of 27% for mid-esophageal lesions, severe intraoperative complications such as massive hemorrhage and tracheal tears are common, leading to few followers. Our department limits the non-thoracotomy approach to stage 0–I lesions with no suspected lymph node metastasis, performing inversion and stripping followed by gastric or colonic reconstruction in the neck. Initially, the indications were strictly controlled, with 5 out of 6 patients (4 stage 0, 2 stage I) achieving long-term survival. Later, it was extended to mid-to-advanced-stage patients unsuitable for thoracotomy, but the outcomes in 25 cases were unsatisfactory, with an anastomotic leak rate of 52% (13/25) and a surgical mortality rate of 12% (3/25). None of the 8 patients with stage IIB or higher survived 5 years.
The median sternotomy with upper mediastinal approach is suitable for resecting high intrathoracic esophageal cancer, though the exposure is inferior to thoracotomy. Another approach involves sternotomy with upward cardiac retraction through the lower mediastinum for cardiac cancer resection. The drawback is suboptimal exposure, affecting the quality of anastomotic suturing. An alternative is using a stapler for mechanical anastomosis.
(4) Selection of alternative organs and transplantation pathways
In principle, a transplanted organ should possess five advantages: good blood supply, high physical strength, good compatibility between the mucosal epithelium and esophageal epithelium, ease of free manipulation, and sufficient length. Practice has shown that the stomach, apart from its poor compatibility, has four strengths and should therefore be the first choice for transplantation. The transplanted stomach occupies part of the thoracic volume, and in the early stages, due to tension-free expansion, it affects cardiopulmonary function, causing discomfort such as shortness of breath and flusteredness in patients. This can be prevented by longitudinally suturing and reducing the stomach. The colon has advantages such as sufficient length and good mucosal compatibility, with moderate blood supply and physical strength. After transplantation, the stomach remains in the abdomen, maintaining better digestive function. However, the procedure is complex, requiring three anastomoses, and the surgical complications and mortality rates are higher than those of gastric replacement of the esophagus, making it the second choice. The jejunum has good compatibility with the esophagus but moderate firmness, and its fragile blood supply affects the length that can be freely manipulated, so it is less commonly used.
The pathways for esophageal replacement include the esophageal bed, intrathoracic, retrosternal tunnel, and subcutaneous anterior chest tunnel. Among these, the esophageal bed has the shortest distance, followed by the retrosternal tunnel, with the subcutaneous anterior chest tunnel being the longest. However, in terms of safety, the subcutaneous anterior chest transplantation method is the safest. In cases of severe complications such as anastomotic leakage or necrosis due to impaired blood supply in the transplanted organ, it is easier to manage and treat because it is located in the neck and subcutaneous area. The retrosternal pathway, like the subcutaneous anterior chest pathway, involves anastomosis in the neck, making it easier to manage if a fistula occurs. Although the intrathoracic pathway is more convenient, if a fistula occurs, it will inevitably lead to empyema, affecting the treatment outcome.
(5) Methods of Esophagogastric Anastomosis
There are various methods, but they can essentially be divided into two categories: two-layer suturing and single-layer suturing. The former can further be divided into full-thickness suturing of the esophageal wall and the transplanted organ wall, and layered suturing of the muscular layer and submucosal layer. Stapler anastomosis belongs to the full-thickness suturing category of the two-layer method. Improved techniques of the two-layer method include tunnel anastomosis, where the anastomosis is reinforced by covering it with the gastric wall, which is similar to the traditional telescope or gastric fundus collar embedding. The embedded esophagogastric anastomosis also falls into the category of reinforcement with the gastric wall. This type of anastomosis has a one-way valve mechanism to prevent reflux of gastric contents.
To avoid the most dreaded postoperative complication, anastomotic leakage, and to establish an anti-reflux mechanism in the newly created anastomosis, numerous improved anastomotic methods have been developed. Some of these have been proven effective through meticulous animal experiments and clinical functional tests. A brief introduction is provided below.
The first is tunnel esophagogastric anastomosis. After resection of the esophageal tumor, the esophageal stump is first fixed to the anterior wall of the gastric fundus. Two parallel transverse incisions, 3 cm apart, are made on the anterior gastric wall about 2 cm from the fixation line. The length of the incisions should allow the esophageal stump to pass through, and the depth should reach the submucosal layer. A tunnel between the seromuscular layer and submucosal layer is dissected between these two incisions. The esophageal stump is guided through the upper transverse incision to the lower incision, where the submucosa is incised, and the esophageal end is anastomosed to the gastric mucosa with single-layer interrupted silk sutures. Finally, the upper edge of the seromuscular flap (the outer wall of the tunnel) is sutured to the esophageal muscular layer, and the lower edge is sutured to the gastric wall to reinforce the anterior segment of the anastomosis.
The second method is called embedded esophagogastric anastomosis. First, one side of the esophageal end is incised to form left and right muscular-mucosal flaps, which are flipped and wrapped around the esophagus and sutured in place. The esophageal stump forms a cone covered with esophageal mucosa. A gastrostomy is made on the anterior wall of the gastric fundus, and the esophageal stump is inserted and sutured to the gastric mucosa.
The third method is called intraluminal elastic ring esophagogastric anastomosis. The esophageal stump is invaginated into an inverted gastric mucosa supported by a stent tube, which presses the outer wall of the esophagus against the seromuscular layer of the stomach. An elastic ring is fixed to the stent tube, and the distal esophagus and gastric wall undergo ischemic necrosis and slough off, while the esophagus and gastric wall heal together.
The fourth type is the sleeve-shaped esophagogastric mucosal anastomosis. The main procedure involves creating a 7-8cm×3cm rectangular seromuscular stripping area on the gastric fundus, carefully preserving the submucosal blood vessels. A 3cm-long mucosal incision is made at the center of this area, and the esophageal stump is resected to retain a 3.5cm-long mucosal sleeve. The posterior (left side for right thoracotomy) wall of the esophagus is sutured to the seromuscular layer at the upper edge of the gastric mucosal bare area. Then, an esophagogastric mucosal anastomosis is performed (using fine silk interrupted sutures), and the absence of fistula or air-fluid leakage is confirmed. The seromuscular layers on both sides of the gastric mucosal bare area are sutured together to form a sleeve covering the anterior (left) wall of the anastomosis. After completion, the adhered esophageal and gastric mucosal "tube" protrudes into the gastric cavity, forming a hollow nipple-like structure.
(VI) Key Points of Surgical Procedures for Esophageal (Cardiac) Cancer
To reduce the incidence of postoperative complications, thoracic surgeons must bear in mind that the surgical treatment of esophageal cancer formally begins from the preoperative preparation stage. All preparations, such as oral care, respiratory care, monitoring of the cardiovascular system, and nutritional supplementation, must be properly completed. The attending physician should have a clear understanding of issues such as the choice of incision, the length of the esophagus to be resected, potential difficulties during resection, and the anastomosis site after resection. During the surgical procedure, the following key points should also be noted.
First, thorough exploration is necessary to determine the length of the lesion, the degree of external invasion, and lymph node metastasis to assess the feasibility of resection and radical treatment. If the lesion has not yet invaded critical mediastinal organs such as the aorta or bronchi, and lymph node metastasis is either absent or limited to a few local metastases that can still be cleared, the diaphragm may first be opened to access the abdomen (via a left posterolateral thoracotomy incision). The abdominal cavity should then be explored for any metastases, followed by mobilization of the stomach for transplantation. After disconnecting the esophagus at the cardiac region and closing the cardiac end, the affected segment of the esophagus is dissected and resected. This approach—understanding the situation first and then proceeding with the main operation—prevents the futile mobilization of the stomach only to find that the esophageal lesion cannot be resected, which would not only be in vain but also subject the patient to unnecessary injury.
Second, whether dissecting the esophagus or the stomach, sharp techniques should be employed as much as possible to ensure complete tumor removal. During the procedure, branches of the esophageal artery, bronchial artery, and left gastric artery must be properly ligated and managed to avoid accidental injury and major bleeding. For mid-segment lesions with significant external invasion involving the aorta or azygos vein, meticulous dissection is crucial to prevent inadvertent injury. In some cases, it may be necessary to sacrifice radicality and leave some cancerous tissue on the vascular wall. If the aorta is accidentally injured, immediate finger pressure should be applied to control bleeding. Due to the high pressure within the aortic wall, atraumatic rat-tooth clamps should not be used to clamp the rupture, as this may enlarge the tear. One approach is to temporarily occlude the aorta with an atraumatic vascular clamp and quickly suture the rupture. Under normal conditions, if occlusion time does not exceed 5–6 minutes, it will not cause damage to organs such as the liver or kidneys. A simpler and more reliable hemostatic method is to mobilize the aorta and wrap the ruptured segment with a split Dacron vascular patch, or to suture a piece of the patient's own muscle tissue over the rupture. When dissecting the posterior and right-sided tissues of the mid-esophagus, direct visualization should be prioritized to avoid injuring the azygos vein. The azygos vein has low internal pressure; if ruptured, the tear can be clamped with rat-tooth forceps and sutured, or the proximal and distal ends can be mobilized and ligated. During gastric mobilization, care must be taken to avoid injuring the splenic artery. In elderly patients, the splenic artery may be tortuous and elongated, forming large loops behind the stomach. When dividing the short gastric vessels, the elongated and curved splenic artery may be accidentally ligated, and the surgeon may only notice when the spleen turns dark purple. In such cases, splenectomy is the only recourse. Another critical step is the ligation and division of the left gastric artery and vein. The surgical field must be fully exposed, and the proximal end should be doubly clamped, suture-ligated, or doubly ligated. After the procedure, careful inspection is necessary to prevent loosening and bleeding. If bleeding occurs, immediate pressure should be applied to control it, the surgical field should be cleared of blood, and the vessel ends should be clearly identified and clamped before secure suture ligation. Blind clamping in panic is strictly discouraged, as it may lead to massive blood loss and endanger the patient's life.
The third key point is to avoid injuring the thoracic duct. When dealing with mid- or upper-segment lesions with severe external invasion, extra caution is required during dissection of the esophagus above and below the aortic arch. Below the arch, the thoracic duct runs between the azygos vein and the descending aorta, posterior and to the left of the esophagus. At the level of the arch, it leaves the vertebral column and crosses to the left side of the esophagus to enter the superior mediastinum. This area is where the thoracic duct is most susceptible to accidental injury, so dissection should be performed under direct vision. After the procedure, the mediastinum should be inspected for any clear lymphatic fluid leakage, which would indicate thoracic duct injury. If present, the duct should be dissected and ligated at its abdominal side in the inferior mediastinum (the direction from which the duct originates). If the dissection is accurate, the leakage should stop immediately.
The fourth key point is to avoid injury to the membranous portion of the left main bronchus. When the upper-middle thoracic lesions involve the anterior wall, they can easily adhere to or infiltrate the membranous portion of the trachea or bronchus. If the dissection is biased toward the tracheobronchial side, it is highly likely to cause damage to the membranous portion, clinically manifesting as significant fistula disease air leakage in the surgical field, making it impossible for the anesthesiologist to maintain adequate positive-pressure ventilation. Once this occurs, timely suturing and repair should be performed, preferably using a pleural flap or muscle tissue to cover and reinforce the area.
The fifth is the prevention of anastomotic leakage. The length of the transplanted organ depends on the transplant site and should, in principle, be sufficient to avoid tension. Tension often affects the blood supply of the transplanted organ due to the influence of the membrane-attached blood vessels. It is known that poor blood supply is one of the significant causes of anastomotic leakage.
Whether a single-layer or double-layer anastomosis method is adopted, the key requirements are full-thickness alignment, appropriate spacing of sutures, and proper tightness of ligatures. This ensures the healing of the anastomosis and keeps the incidence of anastomotic leakage at a very low level. During the anastomosis, if there are concerns about poor healing due to visible local poor blood supply or mismatched esophageal and gastric openings, reinforcement with a chest membrane flap or greater omentum coverage is recommended. Clinical evidence has long proven its effectiveness in preventing fistula formation.
(7) Colon Transplantation as Esophageal Replacement
As mentioned in the discussion of organ selection after esophagus cancer resection, colon transplantation involves extensive preoperative preparation, complex surgical procedures, and higher postoperative complication and mortality rates compared to gastric replacement. However, colon replacement has specific indications in esophageal cancer surgery: ① Lesions in the cervical and mid-thoracic regions; ② Hypopharyngeal cancer resection requiring anastomosis at the floor of the mouth; ③ Inability to use the stomach due to gastric lesions or prior distal subtotal gastrectomy; ④ Extensive cardia cancer requiring total gastrectomy, with colon transplantation serving as a gastric substitute; ⑤ Advanced-stage esophagus cancer where resection is impossible but severe obstruction exists—colon bypass surgery can alleviate symptoms.
The blood supply of the colon originates from the superior mesenteric artery, including the ileocolic artery (supplying the terminal ileum and cecum), the right colic artery (supplying the ascending colon), and the middle colic artery (supplying the hepatic flexure and transverse colon). The inferior mesenteric artery gives rise to the left colic artery (supplying the splenic flexure and descending colon). These arterial branches anastomose to form a complete vascular arcade of the colon.
Due to variations in colonic blood supply, careful evaluation of anastomotic branches is essential before transplantation. In principle, an isoperistaltic colon transplant is preferred if blood supply permits. For example, the middle colic artery may be severed while preserving the left colic artery to utilize the transverse and part of the descending colon. Alternatively, the right colic and ileocolic arteries may be severed while retaining the middle colic artery to use the ascending and part of the transverse colon. If blood supply is insufficient in these scenarios—manifested by the disappearance of distal arterial pulsation after temporary occlusion of the intended severed vessel—an antiperistaltic transplant becomes the fallback option. For instance, severing the left colic artery while preserving the middle colic artery to utilize the transverse and part of the descending colon, or severing the right colic artery while retaining the middle colic artery to use the transverse colon (as the middle colic artery is more right-sided, making antiperistaltic transplantation more convenient). The main drawback of antiperistaltic transplantation is frequent belching, hiccups, and sudden regurgitation of colonic contents (Figure 1).

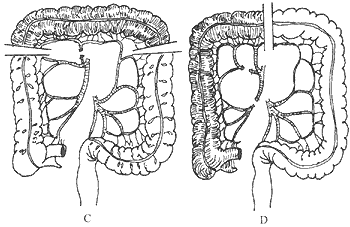
Figure 1 Schematic diagram of colonic blood supply and transplant segments
A. Colonic blood supply: 1. Ileocolic artery; 2. Right colic artery; 3. Middle colic artery; 4. Left colic artery; sm. Superior mesenteric artery; im. Inferior mesenteric artery; B. Severing the left colic artery while preserving the middle colic artery, utilizing part of the transverse and descending colon for antiperistaltic transplantation; C. Severing the middle colic artery while preserving the left colic artery, utilizing the transverse colon for isoperistaltic transplantation; D. Severing the ileocolic artery while preserving the middle colic artery, utilizing the ascending and part of the transverse colon for isoperistaltic transplantation.
During colon transplantation surgery, a left-sided thoracoabdominal combined incision is generally used. When mobilizing the transplant segment, it is required to temporarily block the vessels to be severed, or after the vessels have been severed and the segment is moved upward into the thorax or neck, the distal small stirred pulse of the intestinal tract must exhibit visible pulsation. It often occurs that the blood supply to the intestinal segment is good while it is in the abdomen, but when one end is lifted to the neck, the distal stirred pulse ceases to beat. Although the color change of the intestinal tract may be minimal, careful observation often reveals a slightly darker hue in the intestinal wall, with the membrane and muscular layers showing grade I edema. The pathological change here is actually venous return obstruction in the transplant segment. If this cannot be improved, the transplant segment should be abandoned, and alternative methods for esophageal replacement should be considered. Forcing a suboptimal anastomosis to complete the surgery is highly likely to result in severe complications such as intestinal necrosis postoperatively. The second point to note is the actual usable length of the transplanted intestinal segment. It is not the length of the intestine itself but the length of the vascular arcade of the transplant segment, which limits the height to which the segment can be moved upward. Often, the length of the transplant segment appears sufficient on the surface, but when it is moved upward through the passage (subcutaneous anterior chest, retrosternal tunnel, or esophageal bed), the vascular arcade is found to be insufficiently long, creating tension and preventing the distal end from reaching the intended height. The third surgical key point is that the technique for lifting the transplant segment must be extremely gentle, ensuring the intestinal segment is positioned smoothly. The correct method involves primarily pushing the distal end of the transplant segment upward by hand. Traction on the suture line at the proximal end should be used lightly in coordination with the pushing motion at the distal end. Excessive pulling force on the transplant segment may result in vascular injury, leading to intestinal necrosis. When anastomosing the proximal colon to the esophagus, either an end-to-end or end-to-side anastomosis can be performed. However, since the esophageal lumen is generally smaller and the colonic lumen larger, an end-to-end anastomosis is often difficult to match appropriately. Instead, it is preferable to make a transverse incision on the proximal colon (on the anti-mesenteric side, at least 2 cm from the closed end) that matches the esophageal lumen size, allowing for an easier and more precise end-to-side anastomosis with the esophagus. The distal end of the transplant segment is usually anastomosed to the anterior wall of the stomach near the lesser curvature. A colonic or ileocolic anastomosis is performed to restore colonic continuity. To expedite the surgery, the operating team can divide into thoracic and abdominal groups working simultaneously. The thoracic group opens the chest to mobilize and resect the esophagus and performs the cervical esophageal-colonic anastomosis, while the abdominal group is responsible for mobilizing the colon, performing the colonic-gastric anastomosis, and the colonic or ileocolic anastomosis. The gap in the colonic mesentery should also be carefully sutured to prevent internal hernia formation. During colon transplantation for esophageal replacement, a nasogastric tube may not easily pass into the stomach. In such cases, a small temporary gastrostomy can be created, with a standard gastric tube exiting through the abdominal wall. This serves for early postoperative decompression and, after intestinal peristalsis resumes, as a channel for administering elemental nutritional solutions via tube feeding.
(8) Factors affecting the long-term outcomes of surgical treatment for esophageal cancer
The effectiveness of surgical treatment for esophageal cancer is influenced by multiple factors. Based on literature reports and an analysis of 3,603 cases from the Department of Thoracic Surgery at the Cancer Hospital of the Chinese Academy of Medical Sciences, the confirmed factors include TNM staging, lymph node metastasis, the extent of esophageal cancer invasion, the nature of resection, and the presence of residual cancer at the resection margin.
1. TNM staging: The 5-year survival rates vary significantly among stages. For stages 0–I, it is as high as 83.3%–92.9%; for stage II, 46.3%–53.5%; and for stage III, 6.7%–15.1%.
2. Lymph node metastasis: The 5-year survival rate is 39.3%–47.5% without metastasis and 10%–25% with metastasis.
3. Extent of esophageal cancer invasion: The 5-year survival rate is 34.6%–70.8% without invasion and 22.5%–29.5% with invasion (data from the Department of Thoracic Surgery at the Cancer Hospital of the Chinese Academy of Medical Sciences show an even lower rate of 13.3%).
4. Residual cancer at the resection margin: Data from the Cancer Hospital of the Chinese Academy of Medical Sciences indicate that only cases with invasive cancer are affected, with a 5-year survival rate of 10.3%. For carcinoma in situ, the 5-year survival rate can reach 28.6%, close to the overall group level. Other factors reported in the literature show inconsistent results and lack definitive conclusions. One such factor is tumor length, which was found to correlate with prognosis in the 3,603-case group from the Cancer Hospital. For lesions <3 cm, the 5-year survival rate was 56.6%; for 3–5 cm, 31.0%; and for >5 cm, only 27.5%. The same dataset also revealed that tumor differentiation affects prognosis: the 5-year survival rate was 37.9% for well-differentiated tumors, 20.3% for moderately differentiated, and 15.8% for poorly differentiated. The same data source did not find a correlation between tumor location and prognosis, consistent with some literature reports.
(9) Mediastinoscopic esophagectomy for esophageal cancer
Video-assisted mediastinoscopy (VAT) has become a popular topic in thoracic surgery for the resection of intrathoracic diseases. Its applications now include various lung resections, mediastinal tumor resections, and esophageal cancer resections. One author reported 8 cases of esophageal cancer, where 7 successfully underwent intrathoracic esophageal mobilization, followed by laparotomy and cervical incision for gastric pull-up and anastomosis. The average intrathoracic operation time was 180 minutes, with blood loss of 400–800 ml, and the principles of oncological resection (complete tumor and involved lymph node removal) were reportedly achieved. As an emerging technique, more data are needed to evaluate its advantages and disadvantages. Current limitations include prolonged operation time, high costs, inability to perform in cases of severe pleural adhesions, and uncertainty in meeting oncological principles.
Treatment of complications after esophageal cancer resection
(1) Anastomotic complications
Once an anastomotic leak is diagnosed, timely and aggressive measures should be taken based on the patient's condition, such as re-thoracotomy for anastomotic reconstruction or conservative treatment with adequate drainage of empyema/pneumothorax, enhanced nutrition, and antibiotic support.
Indications for re-thoracotomy include: ① Early detection of the anastomotic leak with mild thoracic infection and no signs of systemic toxicity; ② Sufficient residual stomach length from the initial surgery (if the anastomosis was below the arch) to allow mobilization and re-anastomosis above the arch, or sufficient esophageal length below the arch for re-anastomosis; ③ Good general condition and cardiopulmonary function, enabling tolerance of re-thoracotomy; ④ Large anastomotic leak or partial dehiscence, with low likelihood of spontaneous healing.
Key points of redo thoracotomy for anastomotic reconstruction: ① Thorough debridement of the original anastomotic site's esophageal stump and gastrostomy; ② Suture the original gastrostomy and create a new opening in a non-infected area, such as the gastric fundus adherent to the posterior chest wall; ③ Fully mobilize the thoracic stomach, and if necessary, perform laparotomy to further mobilize the abdominal stomach for upward traction, ensuring the second anastomosis is tension-free; ④ Due to varying degrees of inflammatory reaction in the esophagus and stomach, with tissue edema, congestion, and increased fragility (making sutures prone to tearing), the re-anastomosis must be performed gently with precise alignment and covered with the greater omentum.
When the anastomosis is on the aortic arch and the fistula is large, the only feasible transitional measure is to perform a re-thoracotomy to resect the anastomosis, create an esophageal stoma in the neck, return the stomach to the abdomen, establish a jejunostomy for nutritional support, and drain the thoracic cavity to treat empyema. Once the empyema cavity resolves and the patient's general condition improves, a colon interposition can be performed to restore the patient's ability to eat orally. If the patient is critically ill and unable to tolerate the aforementioned re-thoracotomy procedures, a more conservative yet aggressive approach should be adopted: ① Ensure adequate drainage of the empyema cavity. If necessary, partially reopen the original incision under direct vision to remove loculated empyema septa, achieving full drainage and allowing partial lung re-expansion. ② Provide intravenous or enteral hyperalimentation. ③ Administer high-dose effective antibiotics to control infection. ④ Enhance respiratory care to prevent complications such as mucus plugging, pneumonia, and atelectasis.
Anastomotic aortic arch fistula is an extremely dangerous complication, with an incidence of 0.1%–0.3%. Most cases occur within 2–3 weeks postoperatively. The patient may feel well without any warning signs, then suddenly experience massive hematemesis and rapid death. Another scenario is aortic arch fistula secondary to an anastomotic leak above the arch, which occurs later and results from local infection eroding the anastomosis into the aortic arch. Preventive measures include ensuring the anastomosis does not directly abut the aorta during surgery or using omentum to separate the two.
The causes of anastomotic stenosis are varied: ① Technical factors, such as an overly small gastric opening, excessively tight suturing, overly constrictive fourth-layer wrapping or intussusception of the stomach, or using an undersized stapler. ② Excessive tissue repair response leading to excessive scar formation. ③ Excessive tension at the anastomosis. ④ Reflux esophagitis causing cicatricial stenosis. ⑤ Tumor recurrence. Addressing these causes can reduce its incidence. For cicatricial stenosis, early and repeated endoscopic dilation may provide relief. If dilation fails, surgical revision, reshaping, or re-anastomosis may be considered. For tumor recurrence, reoperation or consultation with radiology for external or intracavitary therapy may be appropriate depending on the case.
(II)Pulmonary Complications
① Correct hypoxemia with 40% O2 under positive-pressure ventilation using IPPB or PEEP, adjusting the end-expiratory pressure to 0.49–0.79 kPa (5–8 cmH2O). ② Alleviate pulmonary interstitial edema by strictly controlling fluid intake (intravenous infusion ≤2000 mL) while administering furosemide (20–40 mg) or ethacrynate sodium (25–50 mg) 4–6 times daily. Additionally, administer salt-poor concentrated albumin (10–25%, 10–20 g) 2–3 times daily. ③ Use high-dose corticosteroids, such as dexamethasone 40–60 mg every 6–8 hours, to act on type II pneumocytes, increase surfactant production, promote alveolar re-expansion, reduce alveolar membrane edema, enhance cardiac function, improve peripheral circulation, stabilize lysosomal membranes, and block α-sympathetic activity to alleviate vasospasm. ④ Administer α-receptor blockers such as phenoxybenzamine (20–40 mg, repeat once afte




