| disease | Megaloblastic Anemia |
| alias | Megaloblastic Anemia |
Megaloblastic anemia is a group of anemias caused by impaired synthesis of deoxyribonucleic acid (DNA), primarily due to a deficiency of vitamin B12 or folic acid in the body, and can also be caused by hereditary or acquired DNA synthesis disorders such as those induced by drugs. The characteristic of this condition is macrocytic anemia, with the appearance of a megaloblastic series in the bone marrow, and giant changes in cell morphology are also seen in the granulocyte and megakaryocyte series, and even in some proliferative somatic cells. These megaloblastic cells are prone to destruction in the bone marrow, leading to ineffective erythropoiesis. Approximately 95% of cases are due to nutritional anemia caused by a deficiency of folic acid or (and) vitamin B12, and in the early stages, it is not uncommon clinically to find cases solely presenting with a deficiency of folic acid or vitamin B12. Nutritional megaloblastic anemia has a regional distribution, being more common in the northwest regions of China such as Shanxi and Shaanxi provinces, with a prevalence rate of up to 5.3%; pernicious anemia, however, is rare in China.
bubble_chart Etiology
(1) Vitamin B12 Deficiency
1. Inadequate intake, increased demand. Deficiency caused solely by inadequate intake is very rare, only seen in long-term strict vegetarians. Increased demand is seen in pregnancy, infants and young children, hemolytic anemia, infection, hyperthyroidism, and malignant tumors.
2. Absorption disorders. This is the main cause of vitamin B12 deficiency. Causes include: ① Lack of intrinsic factor. Seen in pernicious anemia, where antibodies to intrinsic factor exist: blocking antibodies and binding antibodies, the former preventing vitamin B12
from binding to intrinsic factor, the latter able to bind to the intrinsic factor-vitamin B12 complex or to intrinsic factor alone, preventing vitamin B12 absorption. Intrinsic factor deficiency is also seen after total or subtotal gastrectomy and corrosive destruction of the gastric mucosa, with megaloblastic anemia occurring on average 5 years after total gastrectomy, and about 30-40% of subtotal gastrectomy patients having vitamin B12 malabsorption. Rare cases include secretion of inactive intrinsic factor. ② Small intestine diseases, such as small intestine malabsorption syndrome, stomatitis diarrhea, segmental ileitis, post-ileum resection, small intestine lymphoma, and scleroderma. Small intestine lesions often also reduce the absorption of folic acid and iron. Additionally, there is the rare familial selective malabsorption syndrome (Imerslund syndrome). ③ Certain drugs, such as sodium aminosalicylate, neomycin, phenytoin, etc., affect the absorption of vitamin B12 in the small intestine. ④ Broad tapeworm Chinese Taxillus Herb in the upper small intestine, surgical blind pouch formation, and ileum diverticulitis due to bacterial proliferation can all seize vitamin B12 from food, causing reduced absorption. ⑤ Gastrinoma and chronic pancreatitis can cause vitamin B12 absorption disorders, due to the impaired transformation of vitamin B12 and R-binding protein to intrinsic factor binding.3. Utilization disorders. Such as TCⅡ deficiency or the presence of abnormal vitamin B12 binding protein common bletilla tuber application of nitric oxide, can affect the transport and utilization of vitamin B12.
(2) Folic Acid Deficiency
1. Inadequate intake, increased demand. Seen in infants, children, and women during pregnancy. Nutritional deficiency mainly due to insufficient intake of fresh vegetables and animal proteins. Increased demand is also seen in chronic hemolysis, myeloproliferative disorders, malignant tumors, hyperthyroidism, and exfoliative dermatitis. Chronic alcoholic cirrhosis reduces both folic acid intake and storage, and alcoholism reduces folic acid intake.
3. Utilization disorders. Folic acid antagonists such as methotrexate, pyrimethamine, and trimethoprim are inhibitors of dihydrofolate reductase, leading to folic acid utilization disorders.
4. Excessive loss. Such as loss during hemodialysis.
(3) DNA Synthesis Disorders Resistant to Vitamin B12 or Folic Acid Treatment
includes the treatment of many antimetabolites such as 6-mercaptopurine, fluorouracil, hydroxyurea, and cytarabine; certain genetic disorders such as orotic aciduria, Lesch-Nyhan syndrome, deficiency of formiminotransferase or N5-methyltetrahydrofolate transferase; as well as vitamin B6 responsive megaloblastic anemia and vitamin B1 responsive megaloblastic anemia.
[Vitamin B12 and folate metabolism]
(1) Vitamin B12 metabolism Vitamin B12 is a cobalt-containing vitamin, chemically known as cobalamin, synthesized only by certain microorganisms. The vitamin B12 required by the human body is mainly obtained from animal foods such as meat, liver, fish, eggs, and dairy products. The daily requirement for adults is about 2.5μg, and the supply in a general diet far exceeds the requirement. The total amount of vitamin B12 in a normal adult body is about 2~5mg, with about 2mg stored in the liver, so deficiency due to insufficient content in food is extremely rare.
There are two types of metabolically active cobalamins: methylcobalamin and adenosylcobalamin. Medicinal vitamin B12
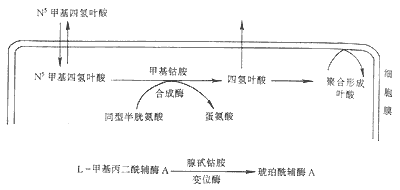
Figure 1 Generation and transformation of vitamin B12 and its relationship with folate metabolism
After being freed from food, vitamin B12 forms a stable complex with the R-binding protein in gastric juice. When the latter enters the duodenum and is digested, vitamin B12 is freed and binds to intrinsic factor. Intrinsic factor is a glycoprotein with a molecular weight of 50000, secreted by gastric parietal cells, proportional to hydrochloric acid secretion. The vitamin B12-intrinsic factor complex can prevent digestion by proteases and enter the distal ileum, binding to the mucosal receptor on the brush border of ileal villi. The bound complex is then taken up into ileal mucosal cells; intrinsic factor is destroyed, and vitamin B12 binds to another transport protein—transcobalamin II. The vitamin B12-transcobalamin II complex is secreted into the bloodstream and can be taken up by liver, bone marrow, and other tissue cells (Figure 2). Although all vitamin B12 absorbed from the intestine is bound to transcobalamin II, most circulating vitamin B12 is bound to transcobalamin I, which is a glycoprotein similar to gastric R-binding protein, partly secreted by white blood cells. This is because vitamin B12 bound to transcobalamin II is cleared very quickly from the blood, while vitamin B12 bound to transcobalamin I takes several days to clear.
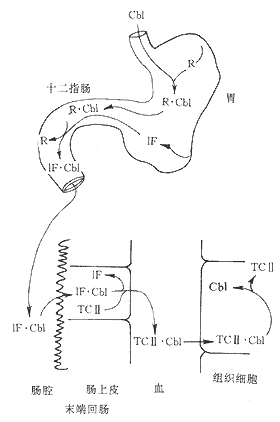
Figure 2 Diagram of vitamin B12 absorption
Cb1 cobalamin 1F intrinsic factor R-binding protein TCII transcobalamin II
(II) Folate Metabolism Folate is a water-soluble B-group vitamin, chemically known as pteroylglutamic acid. Folate is most abundant in fresh green leafy vegetables, and is also found in relatively high amounts in liver, kidney, yeast, and mushrooms. Cooking, pickling, and prolonged storage of food can destroy folate, especially when boiled with water, where the loss is particularly significant. Folate in food exists in the form of pteroylpolyglutamates, which must be hydrolyzed by γ-glutamyl carboxypeptidase in the bile and small intestine into pteroylmonoglutamate and diglutamate before they can be absorbed, primarily in the proximal jejunum. The absorbed folate exists in the blood in the form of N5-methyltetrahydrofolate, loosely bound to albumin for transport, and is taken up into cells via folate receptors. It functions by forming tetrahydrofolate under the action of vitamin B12-dependent methionine synthase (Figure 3); it can also be converted back into polyglutamates for storage, which prevents folate from leaking out of the cells. Adults require 50-200μg of folate daily, stored in liver cells with a storage capacity of only 5-10mg, hence nutritional macrocytic anemia is mainly caused by folate deficiency.

Figure 3 Diagram of Folic Acid Metabolism
Tetrahydrofolate acts as a coenzyme in the transfer of "one-carbon groups" in the body, including methyl (-CH3), formyl (-CHO), methylene (-CH2), methenyl (-CH), and hydroxymethyl (-CH2OH) groups. Serine is a source of one-carbon groups, which reacts with tetrahydrofolate to form N5.10-methylenetetrahydrofolate and glycine; another source is imidazole carboxamide ribonucleotide, which reacts with tetrahydrofolate during histidine catabolism to produce N5-formiminotetrahydrofolate and glutamic acid. These coenzyme forms of folate carry various one-carbon groups and thus participate in some important generation and transformation reactions in the body: such as the synthesis of thymidine nucleotides and purines, the formation of thymidine nucleotides (dTMP) and dihydrofolate from deoxyuridine monophosphate (dUMP) and N5.10-methylenetetrahydrofolate. dTMP is an important raw material for DNA synthesis: ① participating in the synthesis of carbons 2 and 8 in the purine ring; ② participating in the synthesis of methionine, by transferring the methyl group from N5methyltetrahydrofolate to homocysteine, forming methionine (Figure 1).
Vitamin B12 and folic acid are important coenzymes in the process of DNA synthesis in cells. Deficiency of vitamin B12 and folic acid leads to impaired DNA synthesis. The impairment of DNA synthesis due to vitamin B12 deficiency is caused by disturbances in folic acid metabolism (Figure 1). With vitamin B12 deficiency, N5methyltetrahydrofolate in cells cannot be converted into other active forms of tetrahydrofolate, nor can it be converted into polymeric forms of folate to maintain sufficient intracellular folate concentration. Deficiency of vitamin B12 and folic acid leads to a decrease in thymidine nucleotides, slowing down the rate of DNA synthesis, while intracellular deoxyuridine monophosphate (dUMP) and deoxyuridine triphosphate (DUTP) increase. The decrease in thymidine triphosphate (dTTP) leads to the incorporation of uracil into DNA, causing DNA to become fragmented, slowing down DNA replication, and prolonging the nuclear division time (prolongation of S phase and G1 phase), resulting in larger nuclei than normal, with nuclear chromatin appearing as a loose dot-like network, lacking concentration, while the synthesis of RNA and proteins in the cytoplasm is not significantly impaired. With delayed nuclear division and increased synthesis, giant blood cells are formed, with asynchronous development of nucleus and cytoplasm, and loose nuclear chromatin, the so-called "old cytoplasm and young nucleus" changes.
bubble_chart Pathological Changes
The most significant and characteristic megaloblastic changes are observed in the erythroid series, known as the megaloblastic erythroid series. Megaloblasts are large in size with loose chromatin, presenting a punctate reticular structure. The nuclei of megaloblasts are large and blue, while the chromatin in late megaloblasts is poorly condensed, often located at the periphery and may appear lobulated, with the cytoplasm filled with hemoglobin. Mature red blood cells are large and thick, often oval-shaped, lacking a central pallor, and accompanied by varying sizes, polychromasia, or containing basophilic stippling, Cabot rings, or Howell-Jolly bodies.
Megaloblastic changes are also seen in the granulocytic and megakaryocytic series, particularly prominent in late granulocytes. Late granulocytes and band cells are large in size, with swollen and deformed nuclei, loose chromatin, and coarse granules in the cytoplasm, referred to as giant late granulocytes and giant band cells. The nuclei are hyperlobulated, often with more than 5 lobes, even up to 16 lobes, known as giant hypersegmented granulocytes. Megakaryocytes also increase in size with excessive nuclear lobulation, and the nuclei may not be connected. Platelet production is impaired, and large and irregularly shaped platelets can be observed.
The bone marrow shows a hyperplastic appearance, but the blood picture reveals pancytopenia, with the main pathophysiological change being ineffective erythropoiesis, granulopoiesis, and thrombopoiesis, known as intramedullary hemolysis. The lifespan of megaloblasts and macrocytes is shorter than normal, leading to increased serum bilirubin, decreased haptoglobin, and increased lactate dehydrogenase, particularly LDH1 and LDH2 (derived from erythroblasts). Increased serum lysozyme reflects the destruction of granulocytes.
Vitamin B12 also participates in the metabolism of nervous tissue. Deficiency of vitamin B12 reduces methionine synthesis, which in turn leads to impaired synthesis of choline and phospholipid-containing choline, and due to the lack of adenosylcobalamin, results in the accumulation of large amounts of methylmalonyl-CoA and its precursor propionyl-CoA. Abnormal fatty acids synthesized enter neural lipids, leading to demyelination, axonal degeneration, and ultimately neuronal cell death. The nervous system can be affected in the peripheral nerves, posterior and lateral columns of the spinal cord, and the brain.
bubble_chart Clinical Manifestations
The clinical manifestations of vitamin B12 and folic acid deficiency are essentially similar, both can cause megaloblastic anemia, leukopenia and thrombocytopenia, as well as gastrointestinal symptoms such as loss of appetite, abdominal distension and fullness, diarrhea, and glossitis, with glossitis being the most prominent, characterized by a red tongue texture, atrophy of the lingual papillae, and a smooth surface, commonly referred to as "beef tongue," accompanied by pain. Vitamin B12 deficiency is often accompanied by neurological manifestations, such as lack of strength, numbness in the hands and feet, sensory disturbances, difficulty walking, peripheral neuritis, subacute or chronic combined degeneration of the posterior and lateral columns of the spinal cord, the latter being more common in pernicious anemia. Children and elderly patients often exhibit psychiatric symptoms, such as apathy, drowsiness, or confusion. Folic acid deficiency can cause emotional changes, which disappear upon folic acid supplementation. Vitamin B12 deficiency can also affect neutrophil function. The main clinical types are:
(1) Nutritional megaloblastic anemia, primarily due to folic acid deficiency, is more common in the northwestern regions of China, particularly in Shanxi, Shaanxi, and Henan provinces. It often has a history of nutritional deficiency, with little intake of fresh vegetables and very little meat, combined with poor dietary and cooking habits, thus often accompanied by manifestations of complex malnutrition, such as iron deficiency, and deficiencies in vitamin B1, B2, C, and protein. This disease is prevalent during pregnancy and infancy. One-third of pregnant women have folic acid deficiency, and nutritional megaloblastic anemia during pregnancy often occurs in the middle to late stages of pregnancy and postpartum. Infections, alcohol consumption, pregnancy-induced hypertension syndrome, as well as concurrent hemolysis, iron deficiency, and excessive bleeding during childbirth can all trigger this disease. Nutritional megaloblastic anemia in infancy is prevalent in infants aged 6 months to 2 years, especially those fed with lance asiabell root and boiled milk. Maternal malnutrition, concurrent infections in the child, and vitamin C deficiency predispose to this disease, as vitamin C has a protective effect on folic acid from destruction.
(2) Pernicious anemia is caused by atrophy of the gastric mucosa of unknown origin leading to impaired secretion of intrinsic factor and vitamin B12 deficiency. It is prevalent among Scandinavians in Northern Europe. Most cases occur in individuals over 40 years old, with the incidence increasing with age, but there are also a few cases of juvenile pernicious anemia, which may be related to congenital deficiency or abnormality of intrinsic factor and defects in the ileal mucosal receptor. The onset of pernicious anemia may be related to autoimmunity, with about 90% of patients having parietal cell antibodies in their serum, and 60% of patients having intrinsic factor antibodies in their serum and gastric juice. Some may also have thyroid antibodies. Pernicious anemia can be seen in hyperthyroidism, chronic lymphocytic thyroiditis, rheumatoid arthritis, etc. Gastroscopy shows significant atrophy of the gastric mucosa with extensive inflammatory infiltration of lymphocytes and plasma cells. This disease also has a certain genetic relationship, with the prevalence in the patient's family being 20 times higher than in the general population. Combined degeneration of the posterior and lateral columns of the spinal cord and peripheral neuropathy occur in 70-95% of cases and may precede the onset of anemia. There is a significant lack of gastric acid, with no free acid even after histamine injection.
(3) Drug-induced megaloblastic anemia includes drugs that interfere with the absorption and utilization of folic acid or vitamin B12 as mentioned above, as well as antimetabolites. Drug-induced megaloblastic anemia can be divided into two major groups: one group responds to treatment with folic acid or vitamin B12, while the other group does not respond to the aforementioned drugs.
(1) The diagnosis of macrocytic anemia is primarily based on the morphological characteristics of blood cells combined with clinical manifestations. The most prominent features in peripheral blood are an increase in large oval red blood cells and hypersegmentation of neutrophil nuclei. The MCV is often greater than 100μM3, and the MCH is usually greater than 32pg. Hypersegmentation of neutrophil nuclei is characteristic. When more than 3% of neutrophils in the blood have 5 or more lobes, or when neutrophils with 6 or more lobes are found, or when the average number of nuclear lobes in 100 neutrophils exceeds 3.5, or when the ratio of neutrophils with 5 or more lobes to those with 4 or fewer lobes exceeds 0.17, these findings have diagnostic value. Severe cases often present with pancytopenia and a decrease in reticulocytes. The bone marrow shows hyperplasia, with the megaloblastic series accounting for 30-50% of the total bone marrow cells, of which megaloblasts and promegaloblasts can account for more than half. It is important to note that typical megaloblasts may not be found 6-24 hours after the initiation of vitamin B12 or folic acid treatment. Hypersegmentation of neutrophils appears earlier than megaloblasts, and the giant changes in the granulocytic series recover later than megaloblasts after treatment. Megaloblasts are negative for glycogen staining.
(2) The following tests can be used to determine vitamin B12 or folic acid deficiency:
1. The following tests can be used to determine vitamin B12 deficiency:
(1) Serum vitamin B12 measurement: Commonly used methods include the microbiological assay and radioimmunoassay, with the latter having higher sensitivity and specificity and being more convenient. The normal range is 200-900pg/ml, and levels below 100pg/ml indicate deficiency.
(2) Urinary methylmalonic acid measurement: Vitamin B12 deficiency blocks the conversion of methylmalonyl-CoA to succinyl-CoA, leading to an increase in methylmalonic acid in the body and its excretion in large amounts in the urine. Normal individuals excrete only trace amounts (0-3.5mg/24h).
(3) Vitamin B12 absorption test (Schilling test): After fasting, 0.5μg of 57Co-labeled vitamin B12 is administered orally, followed by an intramuscular injection of 1mg of unlabeled vitamin B12 2 hours later. The radioactivity excreted in the urine over 24 hours is measured. Normal individuals should excrete more than 7%, and less than 7% indicates malabsorption of vitamin B12, with pernicious anemia often showing less than 4%. If malabsorption is detected, the test is repeated after 5 days, and 60mg of intrinsic factor is administered orally. If excretion returns to normal, it confirms intrinsic factor deficiency; otherwise, it indicates intestinal malabsorption. If absorption improves after antibiotic treatment, it suggests that bacterial overgrowth is competing with the host for vitamin B12.
2. The following tests can be used to determine folic acid deficiency:
(1) Serum and red blood cell folate measurement: Microbiological assay and radioimmunoassay can be used. The normal serum folate concentration is 6-20ng/ml, and levels below 4ng/ml indicate deficiency; the normal red blood cell folate concentration is 150-600ng/ml, and levels below 100ng/ml indicate deficiency. Red blood cell folate reflects the body's storage status, while serum folate is easily influenced by dietary intake, making the former more diagnostically valuable.
(2) Formiminoglutamic acid (FIGlu) in urine: The excretion test involves administering 15-20g of histidine orally to the patient and collecting 24-hour urine to measure the excretion amount. The normal FIGlu excretion in adults is below 9mg/24H. In cases of folic acid deficiency, the intermediate metabolite of histidine, PIGlu, is impaired in its conversion to glutamic acid, leading to the accumulation of large amounts of FIGlu in the body, which is then excreted in the urine.
3. Other tests helpful in distinguishing folate or vitamin B12 deficiency
(1) Deoxyuridine suppression test: Bone marrow cells or lymphocytes activated by phytohemagglutinin are incubated with deoxyuridine, then tritium-labeled thymidine (3H-thymidine) is added. After a certain period, the amount of 3H incorporated into the DNA of the cell nucleus is measured. Normal bone marrow cells or activated lymphocytes can utilize deoxyuridine to synthesize DNA, resulting in less incorporation of 3H-thymidine (less than 12% of normal controls). When there is a deficiency of folate or (and) vitamin B12, the utilization of deoxyuridine is impaired, leading to increased incorporation of 3H-thymidine. If folate or vitamin B12 is added beforehand to correct the weakened suppression rate, it helps distinguish between folate or vitamin B12 deficiency.
(2) Diagnostic treatment: A trial treatment with physiological doses of folate (0.2mg/d) or vitamin B12 (1μg/d) for 10 days is conducted to observe whether the patient shows improvement in clinical symptoms, an increase in reticulocytes, rapid improvement in megaloblastic morphology, and a rise in hemoglobin, thereby achieving diagnostic purposes. Since physiological doses are used, it helps differentiate between folate or vitamin B12 deficiency.
(3) Nutritional megaloblastic anemia is a gradual process, progressing through reduced reserves of folate or vitamin B12, metabolic abnormalities, and finally leading to deficiency anemia. Understanding its developmental sequence helps correctly interpret the results of various laboratory tests. For example, in folate deficiency: serum folate levels decrease in weeks 2-3; neutrophils show hypersegmentation in weeks 6-8; FIGlU excretion test becomes positive in weeks 13-14; red cell folate levels decrease in week 17; red cells become macro-ovalocytic in week 18; bone marrow cells show megaloblastic changes in week 19; and anemia appears in week 20.
(4) If megaloblastic anemia is combined with iron deficiency anemia, the megaloblastic changes in the erythroid series may be masked and become atypical. Peripheral blood may show two types of red cells, referred to as "dimorphic anemia," but the megaloblastic changes in the granulocytic series are less likely to be masked, aiding in differentiation. In megaloblastic anemia, serum iron, transferrin saturation, and serum and red cell alkaline ferritin are increased; if decreased, it indicates iron deficiency.
bubble_chart Treatment Measures
(1) Supplemental Treatment According to the principle of supplementing what is lacking, an adequate amount should be supplemented until the required storage level is reached. For vitamin B12 deficiency, intramuscular injection of vitamin B12 can be applied, 100μg daily for 2 weeks, then changed to twice a week for 4 weeks or until hemoglobin returns to normal. That is, during the initial 6 weeks of treatment, the total amount of vitamin B12 should be more than 2000μg. Afterwards, switch to a maintenance dose of 100μg per month, or 1mg every 2 to 4 months, but a monthly maintenance dose has a lower chance of relapse. For those with neurological symptoms, the vitamin B12 dose should be slightly larger, and maintenance treatment should be every 2 weeks. Those with neurological symptoms lasting more than a year are difficult to recover. Those with pernicious anemia, gastrectomy, Imerslund syndrome, and congenital intrinsic factor deficiency require lifelong maintenance treatment. For vitamin B12 deficiency, treatment with folic acid alone is contraindicated as it may exacerbate neurological damage. Those with folic acid deficiency can take folic acid orally, 5mg three times a day, or for those with intestinal malabsorption, intramuscular injection of calcium folinate 3~6mg/D until anemia and disease cause are corrected. If it is unclear which deficiency is present, vitamin B12 and folic acid can be used in combination. It is also believed that for nutritional macrocytic anemia, the combination of both is more effective than folic acid alone. After starting supplemental treatment, reticulocytes peak within a week, white blood cells and platelets return to normal within 2 weeks, and anemia is corrected in about 4 to 6 weeks.
(2) Other Adjuvant Treatments If anemia improvement is unsatisfactory after the above treatment, attention should be paid to whether there is concurrent iron deficiency. Severe cases may also experience relative iron deficiency due to the large number of new red blood cells, and iron supplements should be promptly provided. After supplemental treatment in severe cases, blood potassium may suddenly decrease, and potassium should be promptly supplemented, especially for elderly patients and those with pre-existing heart blood vessel disease. Nutritional macrocytic anemia can be supplemented with vitamin C, B1, and B6 simultaneously.
(3) Disease Cause Treatment Efforts should be made to remove the disease cause and treat the primary condition.
Strengthen nutrition education, correct partial eating habits and incorrect cooking practices. Infants should be encouraged to breastfeed, fed appropriately, and have complementary foods added in a timely manner. Pregnant women should consume more fresh vegetables and animal proteins, and folic acid can be supplemented in the late stage of pregnancy [third stage]. In areas with a high incidence of nutritional macrocytic anemia, active promotion of dietary improvements should be carried out. For those with chronic hemolytic anemia or long-term use of antiepileptic drugs, folic acid preventive treatment should be provided. Those who have undergone total gastrectomy should receive a monthly preventive intramuscular injection of vitamin B12.






