| disease | Viral Hepatitis |
| alias | Viral Heptitis |
Viral hepatitis is a common infectious disease caused by various hepatitis viruses, characterized by strong infectivity, complex transmission routes, widespread prevalence, and a relatively high incidence rate. Clinically, it mainly manifests as fatigue, loss of appetite, nausea, vomiting, hepatomegaly, and liver function impairment, with some patients experiencing jaundice and fever. Some individuals may develop urticaria, arthralgia, or upper respiratory symptoms. Viral hepatitis is classified into five types: hepatitis A, B, C, D, and E. Previously, so-called non-A, non-B hepatitis (NANBH) transmitted through blood was referred to as post-transfusion non-A, non-B hepatitis (PT-NANBH), while that transmitted via the fecal-oral route was called enterically transmitted non-A, non-B hepatitis (ET-NANBH). Recent molecular biology studies have confirmed that the pathogens causing these forms of non-A, non-B hepatitis belong to two types: the former is now known as hepatitis C (HC), and the latter as hepatitis E (HE). Most acute hepatitis patients recover within six months, but hepatitis B, C, and D are prone to becoming chronic, with a small number progressing to cirrhosis and very few developing severe cases. Chronic hepatitis B and C are closely associated with the development of primary hepatocellular carcinoma.
bubble_chart Epidemiology
(1) Source of Infection The primary source of infection for hepatitis A is acute patients and subclinical carriers. In the natural history of hepatitis A, subclinical or latent infections are predominant. During outbreaks of hepatitis A, the ratio of latent to overt infections can be as high as 10:1. Hepatitis A patients are most contagious from the late incubation period to 10 days after onset, becoming non-contagious 20 days after the appearance of jaundice. Experimental data indicate that the duration of HAV shedding in feces from hepatitis A patients is not prolonged, with the highest viral concentrations occurring in the late incubation period and the initial stage of illness. By the third week of illness, HAV is rarely detected in feces. Therefore, hepatitis A patients are non-contagious during the convalescent stage, and epidemiologically significant sources are subclinical or latent carriers of hepatitis A.
Both acute and chronic hepatitis B patients, as well as virus carriers, are sources of infection for this disease. Acute patients remain contagious from the late incubation period to 66–144 days after onset. Due to the short contagious period, their significance as sources of infection is less than that of chronic hepatitis patients and virus carriers. Based on hepatitis B testing results in populations worldwide, it is estimated that there are approximately 215 million hepatitis B virus carriers globally, constituting a major source of infection. Chronic patients are also contagious during active phases of the disease. A domestic household survey of 811 chronic hepatitis patients, compared with 811 healthy households as controls, found a secondary hepatitis incidence rate of 2.68% among contacts, versus 1.09% in the control group. Hepatitis B exhibits a familial clustering trend, with an overall HBV infection rate of 62.5% (including HBsAg, anti-HBs, HBeAg, anti-HBe, and anti-HBc positives), significantly higher than the HBsAg-positive detection rate in the general population (10–15%). In households where parents or mothers are HBsAg-positive, the HBV infection rate can reach 87.5%. Among children of chronic active hepatitis and post-hepatitis cirrhosis patients, HBV infection rates are 91.7% and 66.7%, respectively, while children of HBsAg carriers have an HBV infection rate of 14.2%. The contagiousness of HBsAg-positive chronic patients and asymptomatic carriers depends on HBeAg positivity, with HBeAg-positive individuals being highly contagious.Hepatitis C is distributed worldwide without clear geographic boundaries. In Western Europe and the Americas, the HCV infection rate is approximately 0.3%–1.5%, while in the Middle East, it is around 5%. In one Chinese city, anti-HCV testing among blood donors showed a positivity rate of 7.9% (22/279). The highest-risk group for HCV infection consists of blood transfusion recipients. Therefore, chronic hepatitis C patients and HCV or anti-HCV-positive asymptomatic carriers are all sources of infection for this disease .
(II) Modes of Transmission The hepatitis A virus is primarily excreted through the intestines and spreads orally via daily life contact. The disease often occurs sporadically. If collective units fail to isolate patients, disinfect tableware, maintain hygiene in dormitories and toilets, or implement measures such as food inspection and health supervision of food handlers and vendors in epidemic areas, outbreaks of varying scales may occur. Hepatitis A frequently causes outbreaks, mainly through contamination of water or food. In rural areas, small outbreaks are often linked to well water pollution, with cases related to drinking untreated well or river water. Foodborne hepatitis A outbreaks are not uncommon, often caused by asymptomatic food handlers in the incubation period contaminating cooked food through handling. Internationally, there are numerous reports of hepatitis A outbreaks due to consuming raw shellfish contaminated with HAV. For example, in 1983, an outbreak occurred in 19 areas of southeastern England. A survey of 450 patients revealed that 42.6% had consumed clams before falling ill, compared to only 17.5% in the control group. Domestically, in 1978, Ningbo City experienced an outbreak affecting over 2,000 people due to eating contaminated clams. In 1983, Shanghai also saw a hepatitis A outbreak caused by pollution in the origin area of blood clams. In early 1988, a major outbreak in Shanghai was attributed to consuming raw blood clams, with an average incidence rate of 4,082.6 per 100,000. A sampling survey showed that 32.1% of residents ate clams, with a hepatitis A incidence rate of 14–16% among blood clam consumers. The relative risk of hepatitis A among clam eaters was 23–25 times higher than non-eaters, with an attributable risk of 11.5–15.2. Our university’s Department of Epidemiology was the first to isolate HAV directly from blood clams using nucleic acid hybridization and tissue culture methods, later confirming HAV presence in clams via polymerase chain reaction (PCR). This further verified that the outbreak was caused by consuming contaminated blood clams.Hepatitis B virus (HBV) can be transmitted through blood transfusions, plasma, blood products, or the use of contaminated syringes, needles, acupuncture needles, blood collection devices, and even hemodialysis carries a risk of HBV infection. A survey report showed that among 6,950 individuals with a history of injections within six months, the HBsAg positivity rate was 4.65%, while among 10,953 individuals without a history of injections during the same period, the HBsAg positivity rate was 3.99%, with a statistically significant difference between the two groups. Regarding oral transmission, some believe that the virus must enter through breaks in the digestive tract mucosa, such as oral ulcers, gastric and duodenal ulcers, or esophagitis, to enter the bloodstream and cause infection. Under normal digestive tract conditions, the likelihood of oral transmission of hepatitis B is much lower than that of hepatitis A. In foreign experimental infections, 45 "volunteers" were given oral suspensions of feces from hepatitis B patients, and none developed the disease. Epidemiological surveys also indicate that among sanitation workers who have frequent contact with feces, the positivity rates of HBsAg and anti-HBs are no higher than those of control groups or other populations. Additionally, the role of various bodily fluids in the transmission of hepatitis B should not be overlooked. It has been proven that HBsAg can be detected not only in serum but also in saliva, urine, bile, breast milk, sweat, amniotic fluid, menstrual blood, semen, vaginal secretions, pleural ascites, and other fluids. Among these, saliva plays a particularly significant role in transmission, as HBsAg can be detected in 25–50% of the saliva from acute or chronic hepatitis B patients or HBsAg carriers. Since hepatitis B is primarily caused by contact with the blood or secretions of patients or HBsAg carriers, healthcare workers are also at risk of contracting hepatitis B from patients, with dentists and surgeons being particularly vulnerable. For example, a foreign dentist who tested positive for both HBsAg and HBeAg in serum, with electron microscopy confirming the presence of Dane particles in the serum, was found to have transmitted HBV to 11 of his patients over four years, with 9 of them sharing the same HBV subtype as the dentist. The dentist often operated without gloves and had a history of frequent hand injuries, leading to blood-to-mouth transmission of HBV. Neijing Mother-to-child transmission of hepatitis B mainly occurs during childbirth through contact with maternal blood or amniotic fluid and close postnatal contact; however, a small proportion (about 5%) may result from intrauterine infection. HBeAg has been found in the liver tissue of fetuses obtained from induced abortions or late abortions of HBeAg-positive pregnant women, indicating that the virus can be transmitted directly through the placenta. The infection rate among infants of HBeAg-positive mothers exceeds 90%, while for infants of HBsAg-positive but HBeAg-negative mothers, the HBV infection rate is lower (27–30%). Reports from Taiwan, China, and Japan indicate a mother-to-child transmission rate of HBsAg at 36.6–40%. When pregnant women contract hepatitis B during the first trimester (1–6 months), 10% of their infants test positive for HBsAg; if the infection occurs in the third trimester (7–9 months), 76% of infants test positive for HBsAg.Hepatitis C is primarily transmitted through blood transfusions, accounting for over 70% of post-transfusion hepatitis cases. In most developed countries, hepatitis C is the most common type of post-transfusion hepatitis. Prospective surveys in the 1980s (in Europe) showed that 6–12% of individuals who received multiple blood transfusions from volunteer donors developed post-transfusion hepatitis. Foreign reports indicate that among 1,664 blood transfusion recipients, 246 (14.8%) developed post-transfusion hepatitis, with 237 (96.3%) of these cases being hepatitis C. Among kidney transplant patients, most acute and chronic hepatitis episodes are likely caused by hepatitis C.
With the application of immunoassay diagnostic reagents (anti-HCV, HCV-RNA-PCR method), the post-transfusion HCV infection rate has significantly decreased. For example, in the United States, the infection rate dropped from the previous 3.84% to 0.45%. Conversely, lax testing of blood or blood products can lead to outbreaks of hepatitis C. For instance, in a certain region of China, contamination during the process of plasmapheresis and red blood cell reinfusion caused an epidemic of the disease. Follow-up studies over two years later showed that the serum anti-HCV positivity rate among transfusion recipients reached 100%.
The fact that HCV is transmitted through bloodborne infection has been confirmed. However, approximately 40–50% of sporadic HCV infections occur in individuals with no history of blood transfusion or blood product use, and the exact transmission route remains unclear. However, reports indicate that various body fluids—including ascites, pleural effusion, semen, urine, and vaginal secretions—can test positive for HCV using double-amplification PCR techniques. Additionally, the risk of HCV infection is higher among homosexual individuals and those living in close contact with hepatitis C patients or HCV carriers in household or communal settings. Therefore, transmission through close contact should also be taken seriously. Mother-to-child transmission of HCV has been confirmed. For example, among 25 infants born to anti-HCV-positive mothers, 14 tested negative for anti-HCV 2–4 months after birth. However, by 6–12 months after birth, 11 of these infants became anti-HCV positive and developed hepatitis C.
The transmission mode of HDV is the same as that of HBV. Acute and chronic hepatitis D patients, as well as HDV and HBV carriers, serve as the Bingchuan source of infection. HDV infection is unevenly distributed. Italy reports an HDV infection rate as high as 50% among HBsAg carriers, while in Germany and the United States, the rates are only 1.9% and 0.39%, respectively. In China, HDV infection rates vary by region. In Beijing, the anti-HD positivity rate was 2% (5/244 cases), but the detection rate of HDV in liver tissue was 4.76% (2/42) in subacute severe hepatitis, 5.78% (7/121) in chronic active hepatitis, and 5.0% (2/40) in chronic persistent hepatitis. Notably, all 9 HBsAg carriers tested positive for HDV in liver tissue.
Mother-to-child transmission of HDV occurs only in infants born to HBeAg-positive and anti-HD-positive mothers, with most HDV infections occurring during the perinatal period.
The pestilence source of hepatitis E is primarily fecal contamination of water or food by patients, and the pestilence route mainly involves fecal-oral transmission. Based on epidemic patterns, it can be classified into two types: short-term outbreaks, where water sources are contaminated once, leading to epidemics lasting several weeks; and long-term outbreaks, where water sources are continuously contaminated, resulting in epidemics lasting several months. Transmission through food or daily contact often leads to localized outbreaks with clear familial clustering.
The prevalence of this disease is closely related to socioeconomic conditions, hygiene habits, and cultural factors. Outbreaks have occurred in Asia, Africa, and Central America, while sporadic cases have been reported in the UK, the US, France, and Russia. In China, epidemics have also been documented. For example, in 1986 and 1988, over 78,000 people in Xinjiang were affected by a hepatitis E outbreak. The symptoms resemble those of hepatitis A but are clinically more severe, with a higher mortality rate among pregnant women.
(3) Population Susceptibility Hepatitis A primarily affects children and adolescents. In infants, approximately 60% test positive for anti-HAV in serum within the first three months of life, mainly due to passive acquisition from the mother. Anti-HAV levels decline rapidly after six months, making children highly susceptible to hepatitis A. In some developed countries, the prevalence of hepatitis A is relatively low. The anti-HAV positivity rate in the population gradually increases with age, with most individuals over 50 years old already possessing anti-HAV. In contrast, in some developing countries, hepatitis A is highly prevalent, with most infections occurring in early childhood. Surveys of anti-HAV positivity rates in certain regions of China show: Shanghai averaged 51.0%, with over 90.0% positivity in those over 30 and nearly universal positivity in those over 50; Guangzhou reported 62.3%, Beijing 67.0%, and Taiyuan 72.4%, indicating widespread susceptibility to hepatitis A in the Chinese population.
Hepatitis B occurs more frequently in young and middle-aged adults between 20 and 40 years old. Epidemiological investigations during hepatitis B outbreaks have shown that the level of anti-HBs antibodies in the serum directly reflects resistance to HBV. Most of those who contracted the disease during outbreaks were originally anti-HBs positive, while those with high anti-HBs titers were less likely to fall ill. Regions with high anti-HBs positivity rates in the population are often areas with high prevalence of the disease. In these areas, since most people have been infected with HBV and acquired immunity, clinically typical hepatitis cases are relatively rare, while anicteric, protracted, and chronic hepatitis cases account for a high proportion, and HBsAg carriers are also common. Conversely, in populations with low anti-HBs positivity rates, the larger proportion of susceptible individuals makes outbreaks more likely to occur.
Hepatitis C and E are more common in adults. It is now known that HCV is a global pestilence, with approximately 80-90% of post-transfusion hepatitis cases being hepatitis C. Over the past 10 years, more than 30 outbreaks have occurred in 13 countries, affecting the South Asian subcontinent, Southeast Asia, the Asian part of the former Soviet Union, China, North Africa, Central Africa, Jordan, and Mexico, among others. Many of these outbreaks were caused by waterborne contamination. At least 50% or more of these cases were hepatitis E, with the majority of infections occurring in adults.
Hepatitis A virus (HAV) is a small RNA virus with a non-enveloped, icosahedral, spherical particle structure measuring 25–29 nm in diameter. It contains a single-stranded positive-sense RNA genome with a sedimentation coefficient of 33–35S and a molecular weight of 2.25×106 to 2.8×106. The viral genome has been cloned and sequenced, revealing only one serotype and one antigen-antibody system. HAV exhibits strong resistance in vitro, tolerating 50°C for 60 minutes and acidic conditions at pH3. However, it can be inactivated by 100°C for 5 minutes, chlorine at 1 mg/L for 30 minutes, ultraviolet irradiation for 1 hour, or formaldehyde (1:4000) at 37°C for 72 hours. HAV has been successfully cultured in vitro and can proliferate in various cell types, including primary marmoset liver cells, monkey embryonic kidney cells, human liver cancer cells, human embryonic diploid or fibroblast cells, and sheep membrane cells. Cell-cultured HAV generally lacks cytopathic effects, though HAV propagated in human liver cancer cells may exhibit oncogenic properties, making it unsuitable for hepatitis A antigen vaccine production. After serial passage in vitro, minor nucleotide sequence variations may occur, but the amino acid sequences of the capsid proteins (VP) among different strains remain 98–100% identical.
Due to HAV's single antigenic specificity, cross-neutralization tests have not revealed differences among HAV strains. This provides a theoretical basis for using immunoglobulin to prevent HAV infection in individuals exposed to hepatitis A patients early on. Additionally, since HAV has only one neutralizing epitope, synthetic peptides or recombinant DNA vectors corresponding to this epitope can be used to produce genetically engineered hepatitis A vaccines, which have been successfully developed in some countries.
Hepatitis B virus (HBV) is a 42nm DNA virus composed of an outer envelope (HBsAg) and a core (HBcAg). The HBV core consists of DNA, DNA polymerase, HBcAg, and HBeAg. The surface components of the viral particles exist in both spherical (approximately 22nm in diameter) and tubular (approximately 22nm in diameter and 230nm in length) forms, which are the main envelope protein components, referred to as hepatitis B virus surface antigen (HBsAg), as shown in Figure 1. The hepatitis B genome has a negative-sense strand containing approximately 3,200 nucleotides, with four open reading frames: the envelope protein coding region, the core protein coding region, the polymerase coding region, and the X protein coding region. The envelope protein coding region includes Gene S, pre-S1, pre-S2. Gene-S encodes the major protein, HBsAg, while pre-S1, pre-S2, and Gene-S together encode the large protein (containing surface antigen and pre-S1 and pre-S2 proteins). Pre-S1 alone can encode the pre-S1 protein, and pre-S2 alone can encode the pre-S2 protein, and together with Gene-S, it encodes the middle protein (containing surface antigen and pre-S2 protein). The surface antigen, middle protein, and large protein together form the viral envelope. The serum pre-S1 and pre-S2 proteins appear early and are markers of infectivity. The core protein coding region (including Gene C and pre-C) encodes a 312-amino acid polypeptide, P25, through Gene C and pre-C. After cleavage of the pre-C protein, P25 forms the e antigen (P15-18). If pre-C undergoes mutation, P25 cannot be encoded, leading to the disappearance of e antigen in the blood. Therefore, the absence of e antigen does not necessarily indicate the cessation of replication. Gene C can encode an unprocessed core polypeptide, which is then assembled into HBcAg particles. During HBV replication, HBcAg is expressed in hepatocytes but is not detectable as free HBcAg in the serum. However, its specific antibody, anti-HBc-IgM, may be positive, indirectly indicating HBV replication.
During HBV replication, HBV-DNA (deoxyribonuclease) can appear in hepatocytes and serum. The detection of free HBV-DNA in serum indicates a strong marker of pestilence. In chronic hepatitis B patients, the integration of HBV-DNA in hepatocytes is one of the causes that induce hepatocellular carcinoma.
The P region of the HBV gene, also known as the DNA polymerase coding region, spans 2496bp in length and encodes a polypeptide containing 832 amino acids. This enzyme is essential for HBV-DNA biosynthesis. During HBV replication, the activity of DNA polymerase in serum increases, indicating pestilence. However, this enzyme lacks strong specificity, as DNA polymerase activity can also rise during the replication of other DNA nucleic acid-type viruses.
The X gene of HBV is 462bp in length and encodes a polypeptide containing 154 amino acids, known as the hepatitis B X antigen (HBxAg). A positive serum HBxAg also suggests HBV replication and serves as a marker of pestilence. It can activate proto-oncogenes (oncogenes) in the hepatocyte genome and is associated with the development of primary liver cancer.
HBV replication is closely related to the formation of covalently closed circular DNA (cccDNA) molecules, the synthesis of the HBV-DNA negative strand, and the positive strand (Figure 1). Animal experiments have confirmed that during cloning, cccDNA can initiate a replication cycle synthesizing pregenomic RNA even in the absence of other viruses. For example, in duck HBV infection, cccDNA appears before the viral positive and negative strands, serving as the first viral DNA. These results confirm that cccDNA can act as a template for synthesizing pregenomic RNA and mRNA.
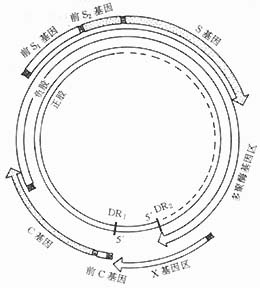
Figure 1. HBV genome structure (black squares mark the starting points of each gene).
The replication and maturation process of HBV in infected hepatocytes primarily relies on the secretion of mature viral particles and the self-amplification of cccDNA in infected hepatocytes. This ensures the long-term stable presence of HBV in infected hepatocytes.
Hepatitis C virus (HCV) can be transmitted through blood. In 1989, Choo et al. in the United States identified a single positive clone out of one million clones from infected chimpanzee blood samples, which was then named hepatitis C virus. HCV is a single-stranded positive-sense RNA virus with an outer envelope, measuring 30–80 nm in size. It can replicate in liver cells, and its infectivity is lost after treatment with 1:1000 formaldehyde at 37°C for hours, heating at 100°C for minutes, or 60°C for hours. To date, the genetic structure of HCV has been clarified. Based on the isolation of numerous HCV gene strains, its complete genome consists of 9,416 base pairs. The nucleocapsid and envelope membrane proteins are encoded by the 5′ end of the genome. The core region of HCV is relatively conserved, while the envelope-coding region is more prone to variation. Foreign scholars have conducted nucleotide sequence analysis of HCV gene strains. Weiner et al. isolated six HCV genomes from hepatitis C patients, among which at least four are globally recognized as major genotypes. Type I is the predominant type in the United States and Europe, namely HCJ-I. Type II includes HCJ, BK, and HcJ4, which is the main type in Japan. Type III is HCJ6, and Type IV is HCJ7. In China, most HCV genes in hepatitis C patients belong to Type II. For example, in Shanghai, among 33 cases of chronic hepatitis C with HCV RNA genotyping, 22 cases (66.6%) were Type II. The analysis of HCV gene sequences provides a theoretical basis for developing reagents for early HCV diagnosis. There are mutation regions within the E1 and E2/NS1 regions, which are important antigenic sites on the viral envelope membrane proteins. The variability of these regions has implications for diagnostic screening, immunoprophylaxis, and persistent HCV infection.
HCV has been successfully cultured in vitro. After inoculating normal chimpanzees with HCV, HCV-RNA can be detected in their serum within 15 days, with a sustained positive duration of approximately 3 weeks. HCV RNA can also be detected in the liver 2 days after HCV inoculation, and anti-HCV becomes positive 3 to 8 months after inoculation.
Hepatitis D virus (HDV) is a defective RNA virus with a diameter of 35–37 nm, an outer envelope of HBsAg, and a molecular weight of 68,000. HDV can exacerbate disease progression and promote chronic infection, and it may be associated with the development of primary liver cancer (HCC). Recent studies using techniques such as serum spot hybridization and in situ hybridization have not only observed that the fluctuations of HDV-RNA align with those of HDAg and anti-HD-IgM and parallel hepatocyte damage but have also yielded new findings. For instance, it was previously believed that HDAg could no longer be detected after the production of anti-HD, indicating viral replication cessation. Coinfection with HDV was thought to suppress the replication and expression of hepatotropic DNA viruses but could worsen hepatitis B progression or lead to fulminant hepatitis, chronic active hepatitis, and cirrhosis. Molecular hybridization studies have shown that about 60% of patients still have low levels of HDV RNA in their blood after the production of anti-HD-IgG, suggesting ongoing viral replication. However, asymptomatic HDV carriers with no significant liver damage have also been identified. In 1989, Luo Shiyuan et al. reported that among 70 cases with positive HBV replication markers, none had detectable HDAg in the liver, whereas 2 out of 37 cases (5.4%) with negative HBV replication markers were positive for HDAg in the liver, which appears consistent with literature suggesting that HDV infection inhibits HBV replication and expression.
The replication mechanism of HDV remains unclear. Some speculate that HDV replication may resemble plant pathology, possibly following a double rolling-circle model for RNA replication.
In chimpanzee experiments with HBV infection, HDAg was detected in the liver 3 weeks after HDV inoculation, appeared in the serum by the 4th week, and anti-HD became positive in the serum by the 9th week. Repeated HDA inoculations showed progressively shortened incubation periods, severe disease progression, a tendency toward chronic hepatitis, and progressive liver damage. Chimpanzees infected with HDV could die within months to years. Additionally, literature reports that immunohistochemical methods detected HDAg in the nuclei and cytoplasm of hepatocytes in woodchucks experimentally infected with HDV, accompanied by typical hepatitis lesions. HDAg appeared in the serum 1–5 weeks after HDV inoculation. These results demonstrate that HDV infection tends to progress to chronic hepatitis, aligning with the clinical and pathological course of chronic HBV infection in humans complicated by HDV coinfection.
HDV infection among HBV carriers can lead to disease progression, exacerbation, and deterioration, resulting in severe consequences. Therefore, preventing and controlling HDV infection holds significant importance in hepatitis management.
Hepatitis E virus (HEV) is a newly identified enterically transmitted hepatitis pathogen, previously known as enterically transmitted non-A, non-B hepatitis virus (ET-NANBV). Virus particles can be found in patient feces, measuring 29–38 nm in diameter, spherical with surface protrusions and indentations, lacking an envelope, and classified as a single-stranded, positive-sense RNA virus with a sedimentation coefficient of 183S and a buoyant density of 1.29 g/cm2. Its nucleic acid target sequence is sensitive to RNase but stable to DNase. The virus-antibody complex is highly unstable at 4°C but insensitive to pH changes.
The nucleotide chain length of the HEV genome is approximately 7500 bases, with a Poly A structure at the 3′ end containing 150–200 adenosines. The 5′ end contains a 27-base non-coding region, which includes three open reading frames (ORFs) (namely ORF1, ORF2, and ORF3). The functions of these open reading frames primarily involve encoding non-structural proteins, neutralizing the negative charge of RNA, and recognizing polypeptide immune responses during HEV infection in humans and animals. However, current understanding of the immunological responses to HEV remains limited and requires further in-depth research.
The above elaborates on the etiological characteristics of five types of viral hepatitis (A, B, C, D, and E). However, other pathogens causing this disease, such as the recently reported GB-type viral hepatitis in the United States and the X-type viral hepatitis in Japan, belong to the seventh newly discovered or yet-to-be-recognized novel strains. Recently, the seventh type of viral hepatitis, namely G-type viral hepatitis, was discovered in Beijing, China. Its etiological characteristics and clinical features, among others, still require further in-depth research by a wide range of medical and scientific researchers.
The pathogenesis of viral hepatitis is quite complex. In the past, it was believed that HAV directly damages liver cells, rarely causing liver cell lesions through immune mechanisms. However, recent literature reports indicate that after HAV invades the human body, the initial stage of infection is a primary non-cytolytic phase, during which HAV replicates and releases extensively within liver cells. By the convalescence stage, viral production decreases, and a large number of mononuclear cells infiltrate the portal areas of the liver, accompanied by grade I necrosis of liver cells and intra-lobular cholestasis. HAV can also be detected in extrahepatic tissues such as abdominal lymph nodes, spleen, and kidneys, with immune complex deposits on the glomerular basement membrane. These phenomena suggest that the pathogenesis of hepatitis A may involve immune mechanisms. After HBV infects the human body, the liver and other organ lesions it causes, as well as the onset and progression of the disease, are not directly caused by the virus itself but are related to the immune status of the host. HBV invades liver cells, replicates within them, and then exits without directly damaging the liver cells. However, it forms specific viral antigens on the surface of liver cell membranes. The virus released into the bloodstream stimulates the immune system (T lymphocytes and B lymphocytes), producing sensitized lymphocytes (cellular immunity) and specific antibodies (humoral immunity). The virus in the bloodstream is recognized by immunologically active T lymphocytes, which proliferate upon sensitization. These sensitized lymphocytes bind to viral antigens on the surface of liver cells, releasing various humoral factors such as lymphotoxins, cytotoxic factors, chemotactic factors, migration inhibitory factors, and transfer factors, ultimately killing the virus and damaging liver cells, leading to necrosis and inflammatory reactions. Patients with strong immune responses may develop acute severe hepatitis (fulminant hepatitis), while those with low cellular immune function are prone to chronic hepatitis or becoming carriers after HBV infection. If immune function is normal and a large number of liver cells are infected, the clinical manifestation is typically acute icteric hepatitis. The mechanisms leading to chronic persistent HBV infection may involve both viral and host factors. Recent data indicate that the liver cell genomes of chronic HBV carriers have integrated HBV-DNA, with longer disease duration correlating with higher integration rates. The integration of HBV-DNA in liver cells is closely related to the development of primary hepatocellular carcinoma. The pathogenic process of HBV requires the participation of host immune cells or antibodies. Specific cellular immune responses are a major factor in the chronicity of hepatitis B, with cytotoxic T cells (Tc) playing a key role in clearing HBV from liver cells. Tc can recognize liver cells with viral antigens on their surface and, with the assistance of macrophages, attack and destroy these cells while eliminating the HBV released during liver cell destruction. If host cellular immune function is low or defective, Tc function is also impaired, preventing the elimination of HBV from liver cells. The efficiency of Tc in clearing intracellular HBV depends not only on the expression of viral antigens on liver cell surfaces but also on the density of HLA (histocompatibility antigens). Reduced HLA expression on liver cell surfaces may be one reason why Tc cannot effectively clear intracellular hepatitis virus antigens. Natural killer cells (NK) and interferon play significant roles in antiviral mechanisms. Chronic hepatitis B patients often exhibit lower NK activity and reduced interferon production. Low interferon activity may be associated with the chronicity of HBV infection.
Antiviral antibodies play a crucial role in terminating HBV infection. During chronic HBV infection, the production of anti-HBs in the body is reduced, thus failing to neutralize circulating HBV and unable to prevent HBV from infecting healthy liver cells. Antibodies against Dane particles (complete HBV) appear to be even more important for clearing HBV. In chronic HBV infection, the level of anti-Dane particle antibodies in the serum is also decreased.
The hepatocellular damage and inflammatory response in chronic hepatitis B are the result of immune cells acting on hepatocytes, with the destruction of infected hepatocytes by Tc being the most significant. Lymphocytes within the liver tissue directly attacking hepatocytes also contribute to inflammatory changes. In vitro experiments by Don et al. found that lymphocytes, particularly T lymphocytes and K cells (killer cells), invade hepatocytes. The former can release lymphotoxins, while the latter exert cytotoxic effects, leading to hepatocyte necrosis such as piecemeal necrosis. Our hospital conducted pathological liver biopsies on 52 patients with chronic active hepatitis, and electron microscopy revealed lymphocytes invading hepatocytes in 2 cases. Pathological examination of these 2 cases showed more pronounced piecemeal necrosis, clinically active disease, and persistently or recurrently elevated ALT levels for several months. After 3 years of follow-up, both cases progressed to post-hepatitis cirrhosis.
The pathogenesis of chronic active hepatitis (CAH) is also associated with autoimmune reactions targeting cell membrane components, primarily manifested by the presence of antibodies against hepatocyte membrane components. These antibodies may directly injure hepatocytes or mediate antibody-dependent cellular cytotoxicity (ADCC), leading to hepatocyte injury. Two specific antigens are present on the hepatocyte membrane surface of CAH patients: LSP (liver-specific lipoprotein) and LMA (liver membrane antigen). Corresponding anti-LSP and anti-LMA antibodies are found in the patients' serum. When anti-LSP enters the liver lobules via circulation, it first accumulates in the periportal area, causing piecemeal necrosis through ADCC.
Serum immunoglobulin levels in CAH patients can be significantly elevated. Comparative observations by Prys et al. revealed that elevated serum IgG in these patients often indicates active intrahepatic lesions and significant hepatocyte necrosis. Elevated IgA is associated with marked intrahepatic fibrosis, while concurrent elevation of IgG, IgA, and IgM suggests severe lobular structural damage and fibrosis. Our hospital measured serum IgE in chronic hepatitis patients and found that CAH patients often exhibit significantly elevated serum IgE. The level of serum IgE activity mostly parallels ALT activity changes and correlates to some extent with the severity of liver lesions. Among 71 cases with elevated serum IgE activity, 68 (95.3%) also showed increased ALT activity. Conversely, among 24 cases with normal serum IgE activity, 21 (87.5%) had normal ALT levels. Patients with elevated serum IgE activity tend to have more frequent and severe focal or piecemeal necrosis. Generally, the increase in immunoglobulins is attributed to reduced Kupffer cell phagocytic function, allowing antigens to spill over into antibody-producing sites (e.g., spleen), leading to excessive autoantibody production. Thorn et al. reported that interactions between specific antigens and tissue-bound IgE, mediated by chemical factors (e.g., histamine, prostaglandins), can lower intracellular cAMP levels, causing cellular damage. Activated mast cells may then release IgE into tissues, elevating serum IgE activity. In CAH patients, impaired Ts cell function may lead to excessive IgE synthesis, which in turn triggers the release of histamine and other bioactive substances by intrahepatic mast cells, potentially contributing to CAH pathogenesis.
Recent studies using monoclonal antibodies against T-cell subsets (CD series McAb technology) to investigate the composition of T-cell subsets in the inflammatory response of liver tissue in chronic hepatitis have shown a decreased percentage of CD4 helper T cells and an increased percentage of CD8 suppressor/cytotoxic T cells (Ts/Tc), resulting in a significant decline in the CD4/CD8 ratio. It has also been confirmed that the suppressor T cells in patients exhibit functional and phenotypic inconsistencies, specifically, impaired suppressor T-cell function alongside an elevated percentage of CD8 suppressor T cells. The immune regulatory dysfunction involves at least two types of suppressor cell impairments and correlates with disease severity. Our university’s pathology department used McAb and the ABC method to perform in situ typing of infiltrating mononuclear cells in liver tissues from 79 cases of various liver diseases. The results revealed that chronic active hepatitis had a higher number of mononuclear cells, most of which were T cells, and that CD8 cells were closely associated with hepatocyte necrosis. This suggests that the parenchymal damage in chronic hepatitis is directly related to CD8 cells.
The relationship between the extrahepatic manifestations of hepatitis B and their corresponding circulating immune complexes (CIC) has been widely recognized, but the role of immune complexes in liver damage remains controversial. A literature review of 108 autopsy cases of various hepatitis and cirrhosis found that HBV antigens (HBVAg) and/or HBsAg immune complexes were present in the liver and extrahepatic tissues in 58.1% of cases. Pathological examination results suggested that the degree of liver damage was proportional to the amount of HBsAg immune complexes in the liver. Ray et al. confirmed that, in addition to immune complexes of HBsAg and its antibodies, immune complexes of HBcAg and its antibodies were also present in the hepatocytes of viral hepatitis patients, but they suggested that the latter may not necessarily cause liver injury.
Recent literature reports have detected various immunomodulatory substances in the serum and liver tissues of hepatitis patients, including rosette inhibitory factor (RIF), very low-density lipoprotein (VLDL), liver immunoregulatory protein (LIP), serum inhibitory factor (SIF), and interleukin-2 (IL-2). Dysfunction of these immunoregulatory substances can lead to hepatocyte destruction, whereas their proper function may alleviate liver damage. In summary, the pathogenesis of hepatitis B is intertwined with immune responses in a complex manner. Currently, most scholars believe that HBV alone cannot independently cause pathological changes, and the disease process must involve host immune responses.
bubble_chart Pathological Changes
The pathological changes of various types of viral hepatitis can be classified into three major categories based on the severity of lesions and the course of the disease: acute, chronic, and severe hepatitis.
(1) Acute Hepatitis: The liver is mostly enlarged with a smooth surface. Microscopic examination reveals the following changes:
1. Active Phase: Hepatocytes show cloudy swelling, edema-like changes, and ballooning degeneration. There is inflammatory cell infiltration in the lobules, predominantly lymphocytes, along with scattered punctate necrosis. Kupffer cell hyperplasia is evident. In jaundice-type hepatitis, cholestasis is observed in hepatocytes and bile canaliculi, and eosinophilic changes may also be seen. In severe cases, cells shrink or even disappear, forming small, red-stained round bodies known as eosinophilic bodies. These active sexually transmitted disease changes can persist for several months.
2. Resting Phase: Focal necrosis, degeneration, and inflammatory reactions in hepatocytes subside, and hepatocyte regeneration and repair are observed, manifested by increased nuclear division or binucleation. Kupffer cells remain swollen.
The pathological changes in acute non-jaundice hepatitis are generally similar to those in jaundice-type hepatitis but are milder, without cholestasis in the capillaries.
(2) Chronic Hepatitis: The liver in most patients is larger than normal, with a medium texture. In chronic active hepatitis, the liver may sometimes appear granular or form nodules.
In China, the histological changes of chronic hepatitis are classified into four types: chronic persistent hepatitis, chronic active hepatitis, chronic severe hepatitis, and cirrhosis.
1. Chronic Persistent Hepatitis: This is further divided into the following three categories:
⑴ Chronic Lobular Hepatitis: The main changes include inflammation within the hepatic lobules and hepatocyte degeneration and necrosis. The changes in the portal area are not significant. Morphologically, it is indistinguishable from mild acute hepatitis and results from the unresolved persistence of mild acute hepatitis lesions.
⑵ Chronic Septal Hepatitis: The inflammatory reaction and degeneration/necrosis within the lobules are mild. Fibroblasts from the portal area extend into the lobules to form septa, which contain few inflammatory cells and do not form pseudolobules.
⑶ Chronic Portal Hepatitis: The degeneration and necrosis of liver parenchyma are mild, with occasional punctate necrosis and rare eosinophilic bodies. The portal area shows significant inflammatory cell infiltration, causing enlargement of the portal area, but there is no destruction of the limiting plate or piecemeal necrosis.
2. Chronic Active Hepatitis: Piecemeal necrosis is the main feature. Lesions within the lobules include punctate and/or focal necrosis, even focal confluent necrosis, as well as degeneration and inflammatory reactions. The presence of bridging necrosis and septal formation indicates more severe lesions.
3. Chronic Severe Hepatitis: Essentially includes severe chronic active hepatitis, where hepatocyte necrosis is more extensive, affecting multiple lobules and disrupting lobular integrity, or presenting as highly active cirrhosis.
4. Cirrhosis: Divided into active and inactive types.
⑴ Active Cirrhosis: Cirrhosis accompanied by piecemeal necrosis, which is observed around the portal area and at the junction of fibrous septa and liver parenchyma. Hepatocytes show degeneration, necrosis, and inflammatory reactions.
⑵ Inactive Cirrhosis: Inflammatory cells are rare in the fibrous septa surrounding pseudolobules, and the boundary between stroma and parenchyma is clear.
(3) Severe Hepatitis: The liver parenchyma is severely destroyed, presenting as massive or submassive necrosis. Based on the disease course and severity of lesions, it is divided into acute and subacute types.
1. Acute Severe Hepatitis The onset is rapid, and the course is short, mostly around 10 days. Due to massive loss and autolysis of hepatocytes, the liver volume significantly shrinks, weighing only 1/3 to 2/3 of the normal liver weight. The texture is soft, and the membrane appears wrinkled. The liver's cut surface shows a blurred structure with alternating red and brown areas, caused by bile stasis in hepatocytes and dilation and congestion of sinusoids. Microscopically, most hepatocytes are lost, with only a small number remaining at the edges of the lobules. The hepatic lobules are infiltrated with monocytes and lymphocytes. The hepatic sinusoids are dilated and congested, with Kupffer cell hyperplasia containing phagocytosed material and pigments. The portal areas and their surroundings show extensive lymphocyte infiltration and proliferation of bile ductules. In cases where the course exceeds 10 days, hepatocyte regeneration and formation can be observed. For those lasting more than 20 days, hepatocyte regeneration becomes pronounced, potentially forming small nodules.
2. Subacute Fulminant Hepatitis The course of the disease ranges from several weeks to several months. The macroscopic pathological changes are the same as those of acute severe hepatitis, but regenerative nodules can be observed on both the surface and the cut section of the liver. Microscopically, submassive necrosis is seen, often appearing as band-like areas affecting the central and intermediate zones of the lobules, frequently extending across lobules to connect with necrotic foci or portal areas of adjacent lobules, forming bridging necrosis. The extent and morphology of hepatocyte necrosis vary in age, with marked collapse of the reticulin framework forming wide septa and fibroblast proliferation, some of which have already collagenized. Inflammatory cell infiltration in the liver is prominent, mainly consisting of lymphocytes and large mononuclear cells. Hepatocyte regeneration is evident, with the formation of pseudolobules. Even after the condition stabilizes, it is prone to progress to chronic hepatitis or cirrhosis.
The histopathological changes of hepatitis C are generally similar to those of hepatitis B, but the following features are more prominent in hepatitis C: ① Fatty degeneration of hepatocytes is more common; ② Hepatocyte necrosis often manifests as scattered small focal areas of fragmented Councilman-like bodies. Electron microscopy confirms the presence of 20–27 nm viral particles in hepatocyte nuclei; ③ The inflammatory reaction in the portal area is pronounced, with abundant lymphocyte and plasma cell infiltration, often accompanied by the formation of lymphoid follicles containing active germinal centers. The inflammation frequently extends to the periportal hepatocytes at the lobular periphery, causing piecemeal necrosis, where swollen and degenerated multinucleated hepatocytes are often observed at the necrotic margins; ④ Abnormalities in bile duct epithelial cells, manifested as hydropic degeneration of small and medium-sized bile duct epithelial cells with stratified arrangement, surrounded by lymphocyte and plasma cell infiltration.
bubble_chart Clinical Manifestations
The incubation period for hepatitis A is 2–6 weeks, averaging about 1 month, while for hepatitis B, it is 6 weeks to 6 months. The incubation period for hepatitis C ranges from 2 to 26 weeks, with an average of 7.4 weeks. Hepatitis C transmitted through blood products or nosocomial infections has a shorter incubation period, generally 7–33 days, averaging 19 days.
Clinically, hepatitis can be classified into acute hepatitis (jaundice and non-jaundice types), chronic hepatitis (persistent and active), severe hepatitis (acute and subacute), and cholestatic hepatitis, based on the presence of jaundice, severity of the condition, and duration of the disease.
(1) Acute Hepatitis
1. Acute Jaundice Hepatitis: The course lasts about 2–3 months and is more common in hepatitis A. During the 1988 hepatitis A epidemic in Shanghai, 85.8% of the 648 hepatitis A cases admitted to our hospital presented with jaundice.
(1) Pre-jaundice Stage: Most cases have a gradual onset, with symptoms such as fear of cold, fever, lack of strength, loss of appetite, nausea, vomiting, liver distending pain, abdominal distension and fullness, constipation, or diarrhea. Some cases exhibit obvious upper respiratory symptoms resembling a common cold. Signs are not prominent in this stage, though some patients may have superficial lymph node enlargement. By the end of this stage, urine darkens, followed by jaundice in the sclera and skin.
(2) Jaundice Stage: Jaundice appears in the sclera and skin, peaking in about a week. Some patients may temporarily exhibit clinical manifestations of intrahepatic obstructive jaundice, with deepening jaundice, pruritus, pale gray stools, and an enlarged, tender liver with percussion pain. About 10% of patients have splenomegaly. Liver function tests show significant abnormalities. This stage lasts about 2–6 weeks.
(3) Stage of Convalescence: Jaundice and other symptoms gradually subside, with marked improvement in energy and appetite. The liver and spleen gradually shrink, and liver function normalizes. Some patients may experience lingering symptoms such as bitter taste in the mouth, liver pain, soreness in the lower back, and abdominal distension and fullness. This stage lasts 2–16 weeks, averaging about a month.
Recent observations indicate that some hepatitis A patients may experience viral relapse (jaundice and elevated transaminases) after recovery from the acute phase, with a few progressing to chronicity. For example, Losnicar reported that among 445 hepatitis A patients, 3.1% (14/445) had a prolonged course lasting up to a year, while 5.1% (23/445) developed post-hepatitis indirect hyperbilirubinemia, with jaundice persisting for up to 33 months. During the 1988 Shanghai hepatitis A outbreak, 5.6% (68/1212) of patients experienced relapse during the convalescence stage.
2. Acute Non-jaundice Hepatitis: This type is more common than jaundice hepatitis, with most cases having a gradual onset. Main symptoms include lack of strength, loss of appetite, abdominal distension and fullness, and liver pain. Some patients may experience nausea, vomiting, dizziness, headache, fever, or upper respiratory symptoms. Most cases present with hepatomegaly, tenderness, and percussion pain, with occasional splenomegaly. Liver function impairment is less pronounced than in jaundice hepatitis. Some cases are asymptomatic and are only discovered during physical examinations, which reveal hepatomegaly, abnormal liver function, or positive HBV markers. The duration varies, with most recovering within 3–6 months. However, some cases may persist and progress to chronicity, as seen in hepatitis B and C.
(2) Chronic Hepatitis
1. Chronic Persistent Hepatitis: Patients with acute hepatitis that does not resolve, lasting over six months, may exhibit symptoms such as lack of strength, loss of appetite, liver dull pain, and abdominal distension and fullness, along with grade I liver function abnormalities or fluctuating results. This condition may persist for months to years.
2. Chronic Active Hepatitis Symptoms and signs persist for more than one year. In addition to common symptoms such as lack of strength, loss of appetite, abdominal distension and fullness, and liver area pain, extrahepatic multi-organ damage symptoms may also occur, such as arthritis, nephritis, colitis, thyroiditis, myocarditis, pleuritis, and dry eye-mouth syndrome. Among these, arthritis and chronic nephritis are more common. The liver and spleen are often enlarged, frequently with tenderness and changes in texture. Liver function remains persistently abnormal or shows significant fluctuations. Some patients exhibit dark skin, progressive splenomegaly, spider angiomas, and palmar erythema. Since the clinical severity of chronic active hepatitis does not always parallel the degree of liver pathological changes, sometimes significant liver lesions may present with mild clinical symptoms and minimal liver function abnormalities. For example, in a group of 122 pathologically confirmed chronic active hepatitis patients, transaminase and γ-globulin grade I abnormalities were observed, yet most patients (90%) showed no obvious symptoms. This scenario is more common in post-transfusion hepatitis C, whereas chronic active hepatitis caused by hepatitis B and D often presents with more typical and severe clinical manifestations.
Chronic hepatitis is divided into two types: chronic persistent hepatitis (CPH) and chronic active hepatitis (CAH), which is a clinical classification made under the circumstance that liver biopsy pathological examination is not widely carried out in most domestic hospitals. If liver biopsy pathological examination is performed on patients with chronic hepatitis, the diagnostic nomenclature of chronic hepatitis should follow the recommendations proposed by the international expert group at the 1994 World Congress of Digestive Diseases, which classifies chronic hepatitis as: ① Chronic hepatitis B: hepatitis caused by HBV infection, lasting for 6 months or more, which may progress to coexist with cirrhosis; ② Chronic hepatitis C: hepatitis caused by HCV infection, lasting for more than 6 months, which may progress to coexist with cirrhosis; ③ Chronic hepatitis D: hepatitis caused by HDV infection, coexisting with HBV infection for 6 months or more, which may progress to coexist with cirrhosis; ④ Chronic sexually transmitted disease hepatitis: hepatitis caused by undetermined or unknown viruses, lasting for 6 months or more; ⑤ Chronic hepatitis: undetermined whether viral or autoimmune, lasting for 6 months or more, possibly caused by hepatitis viruses or related to autoimmunity, but unable to distinguish between the two. In addition, the histological activity index (HAI) liver pathology scoring method by Knodell is used to indicate the degree of liver inflammation activity and fibrosis. Finally, the above types of chronic hepatitis are classified into grade I, grade II, and grade III. The above-mentioned scheme was discussed at the Fifth National Conference on pestilence and Chinese Taxillus Herb parasitic diseases (May 1995) in China, and it was recommended that local regions refer to this scheme and supplement or modify it through practice.
(III) Severe Hepatitis
1. Acute severe hepatitis (fulminant hepatitis) Usually begins as acute jaundice hepatitis, with rapid deterioration of the condition within 10 days, accompanied by the following symptoms: ① Rapid deepening of jaundice; ② Significant bleeding tendency; ③ Liver atrophy, possibly with hepatic odor; ④ Neurological symptoms such as dysphoria, delirium, impaired orientation and calculation ability, drowsiness, and even unconsciousness, with most patients exhibiting cerebral edema; ⑤ Hepatorenal syndrome, oliguria, anuria, and azotemia, etc. Liver function damage is severe, with serum bilirubin exceeding 171 μmol/L, significantly prolonged prothrombin time, and decreased serum cholinesterase, cholesterol, and cholesterol esters. Patients often die due to complications such as gastrointestinal bleeding, cerebral edema, infection, and acute renal failure. The course of the disease generally does not exceed 10–14 days.
2. Subacute severe hepatitis The clinical symptoms are similar to those of acute severe hepatitis, but the course of the disease exceeds 10 days. The main symptoms include progressive deepening of jaundice, bleeding tendency, ascites, liver shrinkage, dysphoria or drowsiness, extreme lack of strength, and significant loss of appetite with persistent nausea and vomiting. This type can also lead to death due to hepatic unconsciousness or hepatorenal syndrome or progress to post-necrotic cirrhosis.
(IV) Cholestatic Hepatitis
Clinically characterized mainly by obstructive jaundice, with symptoms such as lack of strength, cutaneous pruritus, hepatomegaly, and grayish-white stools, but relatively mild digestive symptoms. Liver function tests show elevated direct bilirubin, AKP, γ-GT, and cholesterol, with serum transaminase at grade I elevation or near normal. Jaundice may persist for several months to over a year. Most patients recover, with only a few progressing to gall fel cirrhosis.
bubble_chart Auxiliary Examination
(1) Blood Picture The total white blood cell count is normal or slightly low, with relative lymphocytosis and occasional atypical lymphocytes. In severe hepatitis patients, both the total white blood cell count and neutrophils may increase. Platelets may decrease in some chronic hepatitis patients.
(2) Liver Function Tests There are many types of liver function tests, and appropriate ones should be selected based on specific conditions.
1. Jaundice index and bilirubin quantitative test These indicators may be elevated in jaundice-type hepatitis. Urine tests show increased bilirubin, urobilinogen, and urobilin.
2. Serum Enzyme Assays Commonly used tests include alanine aminotransferase (ALT) and aspartate aminotransferase (AST). Serum transaminases may rise during the incubation period, initial stage of onset, or in asymptomatic infections of hepatitis, aiding early diagnosis. It has been confirmed that AST has two forms: ASTs, found in the cytoplasm of liver cells, and ASTm, located in the mitochondria of liver cells. When extensive liver cell necrosis occurs, serum ASTm increases, so severe hepatitis is primarily characterized by elevated ASTm. Since the half-life of ASTm is shorter than that of ASTs, recovery is also earlier. Persistent elevation of ASTm in acute hepatitis may indicate progression to chronic hepatitis. In chronic hepatitis, persistent elevation of ASTm suggests chronic active hepatitis. Besides viral hepatitis in its active phase, serum transaminases may also rise in other liver diseases (such as liver cancer, liver abscess, cirrhosis), biliary tract diseases, pancreatitis, myocardial disorders, shock, and heart failure. Certain physiological conditions, such as intense physical activity or pregnancy, may also cause transient grade I ALT elevation. Glutathione-S-transferase (GST) rises earliest in severe hepatitis, aiding early diagnosis. Fructose-1,6-diphosphatase, one of the glycogen synthases, is significantly elevated in all types of chronic hepatitis. Serum guanase (GDA) activity correlates with ALT and exhibits organ specificity. γ-Glutamyl transpeptidase (γ-GT) may show grade I elevation in chronic hepatitis and significantly increased enzyme activity in cholestatic hepatitis. Serum alkaline phosphatase (AKP) may rise in biliary obstruction and cholestatic hepatitis. In cirrhosis, serum monoamine oxidase (MAO) isoenzyme MAO3 may increase, whereas in healthy individuals and acute or chronic hepatitis patients, the MAO3 band does not rise, which holds certain significance for early diagnosis of cirrhosis.
3. Cholesterol, Cholesterol Ester, and Cholinesterase Assays In liver cell damage, total blood cholesterol decreases, while obstructive jaundice leads to increased cholesterol. In severe hepatitis, cholesterol, cholesterol ester, and cholinesterase may significantly decline, indicating a poor prognosis.
4. Serum Protein and Amino Acid Assays In chronic active hepatitis, protein electrophoresis often shows γ-globulin >26%, while in cirrhosis, γ-globulin may exceed 30%. However, elevated γ-globulin percentages may also occur in schistosomiasis cirrhosis, autoimmune diseases, myeloma, and sarcoidosis.
Serum prealbumin, synthesized by the liver, is also known as thyroxine-binding protein or vitamin A transport protein. It has a molecular weight of 60,000, a half-life of 1.9 days, and a pH of 8.6. It migrates faster than serum albumin in electrophoresis, hence its name. Its concentration decreases with liver parenchymal cell damage, and the extent of decline correlates with the severity of liver cell injury. In severe hepatitis, its levels may be very low or even near zero. Serum prealbumin levels are reduced in 92% of acute hepatitis and 83.8% of chronic active hepatitis patients, returning to normal as the condition improves. However, levels may also decrease in liver cancer, cirrhosis, and obstructive jaundice, which should be noted.
Detect the ratio of branched-chain amino acids (BCAA) to aromatic amino acids (AAA) in plasma. If the ratio decreases or becomes inverted, it reflects impaired liver parenchymal function and provides reference significance for assessing the prognosis of severe hepatitis and evaluating the efficacy of branched-chain amino acid therapy.
5. Serum Procollagen III (PⅢP) Measurement Elevated serum PⅢP levels suggest the potential formation of hepatic fibrosis. Literature reports indicate a sensitivity of 31.4% and specificity of 75.0%. The normal PⅢP value is <175 μg/L.
(3) Serum Immunological Tests Detection of anti-HAV-IgM is valuable for the early diagnosis of hepatitis A. HBV markers (HBsAg, HBeAg, HBcAg, and anti-HBs, anti-HBe, anti-HBc) are significant for determining HBV infection. Measurement of HBV-DNA, DNA-P, and PHSA receptors is highly valuable for confirming HBV replication in hepatitis B patients. High-titer anti-HBc-IgM positivity aids in the diagnosis of acute hepatitis B. Some researchers have obtained the pre-S1 (pre-S1) and pre-S2 genes through genetic engineering methods. Using histochemistry and solid-phase radioimmunoassay, the localization of pre-S antigens in hepatocytes of acute and chronic hepatitis B patients can be studied. Hepatocytes with HBV replication often contain HBsAg pre-S1 and pre-S2. Anti-pre-S1 and anti-pre-S2 can be detected in serum, with the former appearing during the incubation period and the latter before viral replication ceases. Thus, anti-pre-S1 positivity can serve as an early diagnostic marker for acute hepatitis B, while anti-pre-S2 can indicate hepatitis recovery.
Hepatitis C is often diagnosed by excluding hepatitis A, B, E, and other viruses (CMV, EBV). Positive serum anti-HCV-IgM and/or HCV-RNA confirms the diagnosis.
The serological diagnosis of hepatitis D relies on positive serum anti-HDV-IgM, HDAg, or HDV cDNA hybridization. Detection of HDAg or HDV cDNA hybridization in hepatocytes confirms the diagnosis.
The diagnosis of hepatitis E is confirmed by positive serum anti-HEV-IgM or the observation of 30–32 nm viral particles in feces via immune electron microscopy.
Polymerase chain reaction (PCR) is a highly specific and sensitive new method for detecting viral hepatitis. PCR involves the in vitro amplification of specific DNA under the action of primers, synthesizing millions of identical DNA copies within hours, significantly enhancing test sensitivity and specificity. In viral hepatitis, due to the low viral load in serum, current detection methods are insufficiently sensitive, leading to misdiagnosis. PCR can detect viral loads as low as 104/ml, greatly improving detection sensitivity. Initially applied to hepatitis B diagnosis, PCR is now also used to confirm hepatitis C.
Measurement of immune complexes (IC), complement (C3, C4), IgG, IgA, IgM, IgE, and autoantibodies (anti-LSP, anti-LMA, etc.) provides reference value for diagnosing chronic active hepatitis.
(4) Liver Biopsy Pathological Examination This is highly valuable for diagnosing various types of hepatitis. Through liver tissue electron microscopy, immunohistochemical testing, and observation using the Knodell HAI scoring system, accurate data on the etiology, disease cause, inflammatory activity, and fibrosis degree of chronic hepatitis can be obtained, aiding clinical diagnosis and differential diagnosis.
The diagnosis of various types of viral hepatitis primarily relies on antigen and antibody testing. The diagnosis of hepatitis must also be based on a comprehensive analysis of epidemiological data, symptoms, signs, and laboratory tests. When necessary, a liver biopsy pathological examination may be performed.
bubble_chart Treatment Measures
Generally, a comprehensive treatment approach is adopted, and the vast majority of hepatitis patients can recover their health. The treatment principles primarily involve adequate rest and proper nutrition, supplemented appropriately with medication, while avoiding alcohol, excessive fatigue, and the use of drugs that are harmful to the liver. The treatment methods for various types of hepatitis are as follows.
**(1) Acute Hepatitis** This disease is self-limiting. With early diagnosis, appropriate rest, nutrition, and general supportive therapy, most patients can recover spontaneously within 3 to 6 months. For acute hepatitis patients with severe clinical symptoms or profound jaundice, intravenous administration of hypertonic glucose solution, vitamin C, glucurolactone, potassium magnesium aspartate, or additional Chinese medicinals for clearing heat and draining dampness (modified Virgate Wormwood Decoction) may be considered. For acute jaundice-type hepatitis (type B), adrenal corticosteroids (referred to as hormones) should not be used. In a study of 1,805 acute hepatitis patients, 911 were treated with hormones, while the remaining 894 received only general medications such as vitamins as a control group. After 18–24 months of follow-up, it was found that the hormone-treated group had more cases of disease relapse and progression to chronic hepatitis compared to the control group. Hepatitis A patients rarely progress to chronic hepatitis. Therefore, if deep jaundice (intrahepatic cholestasis) persists despite other treatments, hormone therapy may still be considered. In 1989, our hospital treated 648 cases of hepatitis A, including 12 with deep jaundice, of whom 7 received hormone therapy with significant efficacy. Preferred hormones include hydrocortisone succinate or prednisolone. The former is administered at a dose of 200–300 mg in 500 ml of 10% glucose solution via intravenous drip, with gradual dose reduction every 7–10 days based on liver function improvement. The latter is given at 30–40 mg/day, gradually reduced to a maintenance dose of 5–10 mg/day, with a total treatment course of 2–3 months. All the above patients recovered fully after one year of follow-up, with no relapse observed during the course.
**(2) Chronic Hepatitis** Currently, there is no highly effective treatment for chronic sexually transmitted viral hepatitis. Given that the pathogenesis may be related to factors such as viral strain virulence, the number of infected liver cells, and the patient's immune system response, antiviral drugs, immune function modulation, and medications to improve liver cell function may play a certain role.
**1. Antiviral Drugs** Drugs that inhibit hepatitis B virus include interferon (IFN), vidarabine (Ara-A), adenine arabinoside monophosphate (Ara-AMP), acyclovir, foscarnet, azidothymidine (AET), cyanidanol-3, ribavirin, and the interferon inducer polyinosinic-polycytidylic acid (poly I:C). Among these, the combination of interferon with vidarabine or acyclovir, as well as sequential use of hormones and recombinant α-interferon, has shown relatively better efficacy in eliminating HBV replication markers.
Interferon (IFN) consists of three types: α-IFN, β-IFN, and γ-IFN, which are produced by human leukocytes, fibroblasts, and sensitized lymphocytes, respectively, with α-interferon being the most potent. Its mechanism of action lies in blocking viral reproduction and replication, but it cannot enter host cells to directly kill the virus. Instead, it contacts the cell membrane and induces the production of a special protein within the cell called antiviral protein (AVP). The latter can inhibit the transmission of viral mRNA information, thereby preventing viral replication in host cells. In virus-infected cells, interferon can also induce the production of protein kinase and 2′5′ oligoadenylate synthetase (2′5′AS). Subsequently, 2′5′AS activates an endogenous endonuclease to degrade viral RNA, while protein kinase inactivates the enzymes necessary for ribosomal synthesis, thereby reducing protein synthesis and suppressing viral growth. Under certain conditions, interferon can either inhibit or enhance the function of B cells. For example, high concentrations of interferon significantly suppress antibody responses. In clinical applications, large doses of IFN-α are used to treat chronic sexually transmitted viral hepatitis, leading to improvement or normalization of abnormally elevated serum IgG and IgM levels. This effect is also attributed to interferon's inhibition of B cells, alleviating the overproduction of immunoglobulin antibodies by plasma cells. Regarding its effect on effector cells, interferon can increase the expression of histocompatibility antigen-I (HLA-1), which is crucial for cytotoxic T cells to recognize target cells. Additionally, it has been confirmed that γ-interferon enhances the activity of interleukin-2 (IL-2) receptors, while IL-2, in turn, increases mitogen-stimulated lymphocyte-induced γ-IFN production. Thus, IL-2 and γ-IFN are functionally closely linked and exhibit coordinated effects.
The purpose of using interferon to treat chronic hepatitis B is to eliminate HBV-DNA and HBeAg in the body, induce the conversion of serum HBeAg to anti-HBe, make HBcAg in hepatocyte nuclei disappear, improve liver histopathological lesions, and normalize ALT levels. The efficacy of interferon in treating chronic hepatitis B ranges from 30% to 60%. Based on the author's experience with interferon in recent years, chronic hepati




