| disease | Secondary Mediastinal Infection |
| alias | Mediastinitis |
The cervical fascial plane directly communicates with the anatomical planes and visceral spaces of the superior mediastinum. The structures and planes of the inferior mediastinum similarly connect through the fascia to the upper retroperitoneal region. Infections originating from one anatomical space can directly spread to another through this anatomical pathway. Particularly, infections originating in the neck can spread downward into the mediastinum not only due to gravity but also because of the negative pressure in the thoracic cavity. Anatomically, the potential spaces and pathways for infection spread include the retropharyngeal space, pretracheal space, retrosternal space, cervical vascular sheath, and periesophageal space. In recent years, the increasing use of median sternotomy for cardiovascular surgeries has led to a corresponding rise in postoperative mediastinal infections.
bubble_chart Etiology
The most common causative bacteria of mediastinal infection are staphylococci, such as Staphylococcus albus, Staphylococcus aureus, or Staphylococcus epidermidis. Others include gram-negative enterobacteria, such as Enterobacter aerogenes, Alcaligenes, Proteus, Bacillus capsulatus, and Pseudomonas aeruginosa. In recent years, due to the routine use of broad-spectrum antibiotics, Staphylococcus aureus has become less common, and bacterial cultures of pus often yield negative results. In cases of chronic infection, fungi such as Candida albicans are frequently found.
Rupture of organs within the mediastinum
Different causes leading to rupture of the esophagus, trachea, and bronchi can result in suppurative mediastinal infections.
Acute upper mediastinal infections are often caused by injuries to the cervical or thoracic esophagus, such as iatrogenic instrument injuries during esophagoscopy or perforation and erosion of the esophageal wall by foreign bodies. Previous rigid metal tube endoscopy was more likely to cause such perforations.Postoperative thoracic esophageal-gastric anastomotic leaks are also a common cause of acute mediastinal infections, although such inflammation often rapidly spreads into the thoracic cavity, overshadowing the issue of acute mediastinal infection. Severe vomiting inducing the expulsion of pathogens from the exterior, leading to spontaneous esophageal rupture, primarily results in fatal mediastinal infections.
Spread downward through the pretracheal space, peripharyngeal space, or prevertebral space can cause upper mediastinal infections. Because the neck is superficial, drainage and antibiotic treatment are easier to control, so progression from cervical cellulitis or acute lymphadenitis to acute upper mediastinal infection is also a cause of mediastinal infections. Intrathoracic suppurative sexually transmitted diseases, such as empyema or lung abscesses adjacent to the mediastinum, can occasionally spread directly into the mediastinum. Infections ascending from acute suppurative pericarditis or retroperitoneal infections leading to mediastinal infections are extremely rare. In cardiac surgery, particularly in cases with a median sternotomy, postoperative tracheostomy in the philtrum—due to the separation of the suprasternal fossa during surgery—can connect the tracheal incision with the retrosternal space, allowing tracheal secretions to flow into the mediastinum and cause infection. This has been frequently reported clinically.
bubble_chart Clinical ManifestationsThe main clinical manifestations of secondary mediastinal infection are fever, pain, and purulent secretions. The patient's postoperative body temperature does not subside and rises above 39°C within 1 week or rises again after subsiding. Before the onset of remittent high fever, chills may also occur. Incision pain intensifies, and eventually, purulent secretions appear at the local incision or drainage site, with the shortest onset being 3 days postoperatively and the longest exceeding 2 weeks, typically around 7 days. Physical examination reveals tenderness upon pressing beside the incision or along the sternal margin. If the mediastinal infection has spread to osteomyelitis and the sternum becomes unstable, signs of sternal dehiscence may appear. Blood tests show a significant increase in white blood cell count and polymorphonuclear cells. The count may rise to (10–20) × 109/L (1000–2000/mm3) or higher, with some cases reaching above 30 × 109/L (3000/mm3); polymorphonuclear cells often exceed 90%. Lateral sternal radiographs show increased density in the retrosternal shadow, and if osteomyelitis is present, osteoporosis and bone destruction may also be observed.
The diagnostic method is based on the aforementioned medical history. Shortly after esophagoscopy or penetrating mediastinal injury, symptoms such as high fever, shivering, collapse, and shock may rapidly appear, along with severe retrosternal pain, dyspnea, and tachycardia. If there is injury to the trachea or esophagus, early signs may include subcutaneous emphysema and crepitus in the neck. The subcutaneous emphysema typically begins in the cervical region and quickly spreads throughout the body. White blood cell counts may be elevated to varying degrees, with some cases reaching above 30×109
/L (3000/mm3). When acute mediastinal infection spreads to the bilateral hilar regions, significant interscapular pain may occur.If sternal instability develops, patients often complain of exacerbated pain at the anterior chest incision, particularly during coughing, expectoration, or violent thoracic movements, and may experience a sensation of sternal friction or displacement. In the absence of infection, fever is generally absent. When sternal dehiscence is pronounced, superficial skin fissures may appear. If the incision fully dehisces, the two halves of the pectoral muscles may exhibit significant movement during respiration, sometimes overlapping and interfering, impairing respiratory function and leading to symptoms such as tachypnea and tachycardia. During physical examination, early palpation of both sides of the sternum may reveal pectoral muscle movement during deep breathing. In more severe cases, sternal crepitus may also be audible. If there is a small superficial fissure, small air bubbles may be observed being expelled from the mediastinum during deep inspiration or coughing. In cases of complete dehiscence, exposed mediastinal tissues, pericardium, or even the heart may be visible through the wound.
In summary, if a patient exhibits sternal friction or displacement upon palpation during deep breathing after cardiac surgery involving a median sternotomy, a diagnosis of sternal dehiscence can be made. After open-heart surgery, if body temperature rises above 39°C and persists for 4–5 days without improvement or recurs after a temporary drop, careful examination of the incision is warranted. If significant tenderness along the sternum, sternal mobility, purulent discharge, or air bubbles are observed, a diagnosis of mediastinal infection can be confirmed. Radiographic findings of retrosternal shadowing or pneumomediastinum may also aid in diagnosis. If necessary, mediastinal aspiration or subxiphoid puncture may yield purulent discharge.
bubble_chart Treatment Measures
Acute mediastinal infection requires immediate and vigorous measures. Any delay often results in irreparable complications or even rapid death. For example, esophageal perforation contains many highly harmful bacteria from the oral cavity, leading to severe intoxication. Bacteria or toxins are rapidly absorbed through the rich lymphatic network of the mediastinum, quickly causing bacteremia, toxemia, and sepsis.
Once the diagnosis of sternal dehiscence after cardiac surgery is confirmed, reoperation should be performed immediately. If the skin is intact and uninfected, simply reopening the original incision, tightening the loose wires to stabilize the sternum, and then resuturing the subcutaneous tissue and skin is sufficient. If the wires are broken or have cut through the sternum, they should be removed and replaced with new wires to rewrap and tighten the sternum. If one side of the sternum is fragmented or fractured, alternative suturing methods, as shown in Figure 1, should be employed to ensure reliable and stable approximation of the separated sternum.
Figure 1: Fixation method for sternal separation
1. **Open drainage** This is the traditional method, involving opening the incision, irrigating the wound, removing pus, necrotic soft tissue and bone, loose wires, and infected granulation tissue. The wound and mediastinum are then drained with wet gauze, frequently changed, and antibiotics are used to control infection. Once clean granulation tissue appears, the incision is sutured in the intermediate stage (second stage) or allowed to heal by secondary intention. The advantages of this method are the absence of dead space due to poor drainage and the ability to address infection sites promptly. The disadvantages are: ① Significant patient discomfort and prolonged recovery; ② Sternum mobility and chest instability, leading to respiratory failure or pulmonary complications; ③ Prolonged exposure of the sternum, mediastinal tissues, and heart increases the risk of secondary infection of cardiac sutures and prosthetics, often resulting in major hemorrhage from the incision or endocarditis. Therefore, open drainage has a high failure rate and is currently only suitable for cases of mediastinitis occurring 2–3 weeks postoperatively with a relatively stable chest, or for severely ill patients with osteomyelitis who cannot tolerate anesthesia for reoperation.
2. **Closed drainage** Once sternal dehiscence with mediastinal infection is diagnosed, immediate reoperation is performed for thorough debridement, removal of infected mediastinal tissue and fibrous deposits, and primary closure of the incision. Postoperatively, continuous irrigation with antibiotic solution or povidone-iodine solution is administered. This method rapidly controls infection and is highly effective when performed early before the infection spreads to cause sternal osteomyelitis. It is widely regarded as the preferred treatment. Our hospital has successfully treated 17 cases using this method. The specific procedure is as follows:
Reoperation was performed under intravenous ketamine and general anesthesia. Tracheal intubation was used for assisted ventilation. The sutures of the median sternotomy incision were removed, and the original incision was reopened. All suture knots were excised, and any loose, broken, or cut stainless steel wires were extracted. Bone wax applied to the sternal edges was scraped off. Bleeding points were cauterized, with silk ligatures avoided as much as possible. The sternum was retracted, and pus in the mediastinum was aspirated. If the pericardium was closed and free of infection, it was preferable not to enter the pericardial cavity to prevent spreading infection. If pus or an abdominal mass was present in the pericardium, the pericardial sutures were removed to open the pericardial cavity. The cavity was irrigated copiously with normal saline, povidone-iodine, metronidazole, or antibiotic solutions, while purulent fibrinous deposits on the mediastinum, heart, and great vessels were cleared. After thorough debridement, the opened pericardium was left unsutured. A small incision was then made beside the suprasternal notch in the neck to introduce a 0.3 cm diameter silicone tube with multiple side holes, which was placed in the upper mediastinum, with its distal end connected to an irrigation bottle line. Additionally, a latex drainage tube was placed near the diaphragmatic surface of the pericardial cavity and beside the right atrium, with its distal end exiting below the incision and connected to negative-pressure suction [-1.18 to -1.57 kPa (-12 to -15 cmH2O)]. The sternum was then firmly approximated and sutured with stainless steel wires, avoiding the original intercostal spaces. Finally, the skin and subcutaneous tissues were closed in full-thickness sutures.
Before the end of the surgery, continuous irrigation with antibiotic solution should be initiated. Gentamicin is generally used first, with 80,000 units added to every 500 ml of normal saline, or metronidazole can be used. Later, other sensitive antibiotic dilutions such as kanamycin, cephalosporin, ampicillin, or polymyxin can be substituted. The volume of antibiotic solution for irrigation is 1,500–2,000 ml/day. During the irrigation process, the drainage tube must remain unobstructed to prevent blockage by fibrin. At the same time, systemic administration of sensitive antibiotics and supportive therapy should be employed. In most cases, after 3–5 days of irrigation, the drainage fluid gradually changes from turbid to clear, the drainage and irrigation volumes tend to balance, body temperature progressively decreases, and the patient's overall condition improves. Irrigation is usually discontinued within 7–10 days. The silicone rubber infusion tube is removed first, followed by the latex drainage tube 1–2 days later. The wound mostly heals after initial-stage suture removal. The advantages of this method are: ① No sternal displacement occurs, ensuring good sternal stability and respiratory function. ② The patient experiences less pain and no psychological distress from sternal retraction. The healing time is short. ③ It reduces the risk of reinfection due to repeated dressing changes and minimizes bleeding from the heart and major vessels. The disadvantage is the potential for dead space formation due to inadequate drainage (Figure 2).
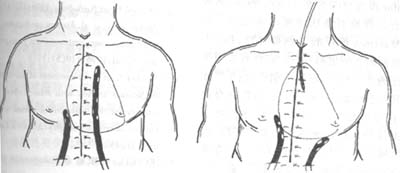
Figure 2 Closed drainage
3. Muscle Flap Technique For patients with mediastinal infection involving the sternum, where the sternum is severely infected or even necrotic, partial or complete sternal resection can be performed. Concurrently, the pectoralis major and rectus abdominis muscles can be partially detached and used to fill the gap left by sternal resection, followed by initial-stage suturing. Pairoler RC reported 38 cases, including 17 with sternal resection and 37 with pectoralis major reconstruction and initial-stage chest closure. Among them, 33 survived, and although 5 died, their deaths were unrelated to sternal infection, demonstrating favorable outcomes. The advantages include a short healing time, good thoracic stability, preserved respiratory function, avoidance of complications from dressing changes, and reduced psychological trauma. In one case at our hospital, a patient with chronic mediastinal infection underwent debridement and partial sternal resection, followed by pectoralis major muscle flap placement and initial-stage suturing, resulting in satisfactory healing. This method is particularly suitable for chronic, recurrent cases, as illustrated in Figures 3 and 4.
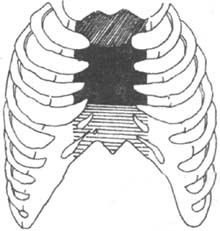
Figure 3 Sternal resection
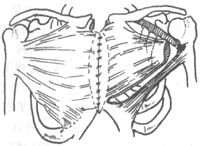
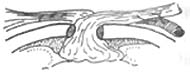
Figure 4 Muscle flap technique
Therefore, once sternal dehiscence with mediastinal infection is diagnosed, immediate surgery is required. If there is no sternal dehiscence or the infection is mild, local wound care may suffice. However, if the sternum or anterior mediastinum is involved, thorough debridement and sternal refixation are necessary. After debridement, drainage tubes should be placed for antibiotic irrigation. For advanced-stage sternal infections, surgical removal of infected sternal tissue, complete debridement, and pectoralis major muscle flap implantation are required.
1. The sternum must be split precisely. Among 17 cases in our hospital, 3 cases had deviated sternal splitting, resulting in transverse sternal fractures.
2. Inadequate intraoperative hemostasis, excessive use of bone wax or electrocautery, poor drainage tube placement, abdominal mass due to blood clots, and re-sternotomy all increase the risk of infection.
3. Fixing the pectoralis muscle reliably by passing steel wires through the parasternal intercostal spaces requires no fewer than 5–6 wires in adults. Culliford suggests placing 2 wires at the manubrium and 4 wires in the parasternal intercostal spaces for optimal results.
4. The pectoralis major fascia should cover the steel wires and the sternal gap.
5. The pericardium should be sutured as much as possible to prevent infection from invading the pericardial cavity, which could lead to severe cardiac bleeding.
6. For patients with severe cough or sputum production, if sternal dehiscence is suspected, a chest binder should be applied for stabilization.
7. Tracheostomy is generally performed 5–7 days postoperatively, and care should be taken to prevent wound infection after the procedure. {|106|}




