| disease | Acute Empyema |
| alias | Pyothorax |
Pathogenic bacteria enter the pleural cavity, causing infectious inflammatory exudation and resulting in inflammatory or purulent pleural effusion, which is called empyema. It is one of the common thoracic diseases, with a higher incidence among young and middle-aged adults. With the continuous development of medical and health services and the widespread use of antibiotics, the incidence of empyema and its complications has significantly decreased. However, the diagnosis and treatment of empyema caused by complex or special drug-resistant bacterial infections, as well as in pediatric and elderly patients, can still be challenging at times. These cases often have a prolonged course, causing long-term suffering, impairing labor capacity, and even leading to death.
bubble_chart Etiology
Pleural infections are primarily secondary infections, with pathogens often originating from intrathoracic organs such as the lungs or esophagus, with the vast majority stemming from pulmonary diseases.
Pulmonary infections like pneumonia can directly invade the pleura or allow pathogens to enter the pleural cavity when the lesion ruptures, leading to acute empyema. Common causative bacteria include pneumococcus, streptococcus, and Staphylococcus aureus. In children, Staphylococcus aureus empyema is more prevalent.
Other common pathogens include Gram-negative bacilli such as Escherichia coli, Proteus, Aerobacter, and Salmonella. Subcutaneous node bacilli and fungi are relatively rare.
Rupture of a lung abscess often results in pyopneumothorax or even tension pyopneumothorax, potentially forming a bronchopleural fistula and leading to mixed infections. If anaerobic bacteria are involved, a putrid empyema may develop, characterized by necrotic tissue in the pus and a foul odor.
Surgeries involving the esophagus, trachea, bronchi, or lungs are considered contaminated rather than sterile procedures. Inappropriate postoperative antibiotic use can still lead to infections and empyema. Postoperative complications like esophageal anastomotic leaks or bronchial stump fistulas further increase the risk of empyema.Spontaneous pneumothorax caused by ruptured pulmonary bullae is usually non-infectious. However, secondary infections leading to empyema may occur during treatment if repeated thoracocentesis or prolonged closed drainage is performed.
In cases of chest trauma, pathogens or foreign bodies such as clothing fragments, bone chips, bullets, or knife tips can be introduced into and retained in the pleural cavity, easily resulting in empyema. Open chest wounds or injuries to the esophagus, bronchi, or lungs that connect the pleural cavity to the external environment can also cause empyema.
Liver abscesses, subphrenic abscesses, and perinephric abscesses can directly invade the pleura or rupture into the pleural cavity, or spread via lymphatic drainage, leading to pleural infections and empyema.
During sepsis or septicemia, pathogens can enter the pleural cavity via the bloodstream, forming suppurative lesions and causing empyema. Particularly in infants, elderly patients, or those with weakened immunity, empyema often represents part of a systemic infection, with severe conditions and poor prognosis.
Spontaneous esophageal rupture or secondary infection of mediastinal teratomas rupturing into the pleural cavity can also lead to empyema.
bubble_chart Pathological Changes
After pathogenic bacteria enter the thoracic cavity, they cause inflammatory changes in the tissues, leading to congestion and edema of the visceral and parietal pleura, which lose their glossiness and lubricity. A thin, clear serous fluid exudes, containing white blood cells and fibrin, but with fewer cellular components. This stage is known as the exudative phase. If effective treatment is administered at this time and the accumulated fluid is promptly drained, the lung can fully re-expand, with minimal impact on pulmonary function.
If timely and effective treatment is not provided during the exudative phase, the inflammation continues to progress gradually. The exudate, fibrin, common bletilla tuber, neutrophils, and even pus cells gradually increase, turning the fluid from clear to turbid and eventually purulent. Fibrin deposits on the surfaces of the visceral and parietal pleura, forming a fibrinous membrane. This stage is known as the fibropurulent phase. The fibrinous membrane is soft and fragile but gradually organizes and becomes tougher, leading to pleural adhesions and localizing the empyema, forming a localized or encapsulated empyema. Although lung expansion is restricted, the impact on respiratory and circulatory functions is relatively minor. Localized or encapsulated empyema can occur between lung lobes, above the lung base and diaphragm, in the posterolateral thorax, or near the mediastinum. If the infection remains uncontrolled and continues to spread, involving the entire thoracic cavity, it develops into a total empyema. The accumulated fluid compresses the lung tissue, causing collapse, and shifts the mediastinum toward the healthy side, resulting in respiratory and circulatory dysfunction. If combined with a bronchopleural fistula or esophagopleural fistula, it forms a pyopneumothorax, further exacerbating the impact on respiratory and circulatory functions.Different pathogenic bacteria produce pus with varying characteristics. Pneumococcal empyema typically yields yellow or yellowish-green pus, which is thick and contains abundant fibrin, making adhesions more likely. Empyema caused by hemolytic streptococci produces thin, light-yellow pus with little fibrin and mild pleural adhesions, making it less likely to localize. Staphylococcal empyema produces thick, yellow pus, sometimes paste-like, with abundant fibrin, leading to rapid and severe adhesions, often forming multiloculated abscesses. Pseudomonas aeruginosa empyema produces green pus. Empyema caused by large intestine bacilli or fecal alkaligenes produces thin pus with a fecal odor, severe tissue necrosis, and a tendency to spread, often resulting in total empyema. Anaerobic streptococci, fusobacteria, and spirochetes cause putrid empyema with a strong, foul odor. Gas-producing bacteria often lead to pyopneumothorax.
With effective antibiotic treatment and timely drainage of pus, acute empyema can gradually resolve, leaving only residual adhesions and pleural thickening in the pleural cavity. If timely and effective treatment is not provided, acute empyema may progress to chronic empyema. Large amounts of fibrin from the pus deposit on the pleura, and capillaries and fibroblasts grow into the fibrin, forming granulation tissue that organizes into a thick, dense membrane—the pleural fibrotic plate. This stage is known as the organizing phase. Extensive, rigid pleural fibrotic plates encase the lung tissue, severely restricting thoracic movement, causing inward invasion of the chest wall, mediastinal shift, and significant impairment of respiratory function.bubble_chart Clinical Manifestations
Acute empyema secondary to pulmonary infection often manifests with symptoms such as high fever, chest pain, dyspnea, cough, general lack of strength, and loss of appetite after the initial improvement of pulmonary infection symptoms. Patients often exhibit an acute sexually transmitted disease appearance, unable to lie flat or change positions without coughing. In severe cases, cyanosis may occur. The affected side shows weakened respiratory movement, with full and widened intercostal spaces. Percussion reveals dullness and tenderness on the affected side. If the effusion is on the left, the cardiac dullness border becomes indistinct; if on the right, the hepatopulmonary border becomes unclear. The mediastinum and heart shift toward the healthy side, and the trachea deviates to the healthy side. Auscultation reveals weakened or absent breath sounds or tubular breath sounds on the affected side, along with weakened vocal fremitus.
The positive signs of localized encapsulated empyema are often atypical, with only certain positive signs present locally in the affected area, making it difficult to detect.
bubble_chart Auxiliary Examination
CT scan: Empyema appears as an arcuate, uniform dense shadow parallel to the chest wall. Changing the patient's position can determine whether the fluid is mobile. A large amount of fluid entering the pulmonary fissure can compress and displace the lower lung inward and backward. When a large amount of fluid is adjacent to the posterior border of the right lobe of the liver, CT scanning shows a blurred posterior border of the right lobe of the liver with indistinguishable boundaries. This is a characteristic change of pleural effusion, known as the "interface sign."
Ultrasound: In the early stages, when there is no fibrin deposition causing thickening of the pleura, the fluid has no sediment, and the anechoic area appears clear without echogenic spots. When there is a large amount of effusion, the lung tissue is compressed, and the gas within the lung is absorbed. Ultrasound may reveal a triangular dense shadow within a large anechoic area, which floats with respiration. When the probe is near the diaphragm, a curved echogenic band representing the diaphragm can be seen, forming a wedge-shaped angle with the chest wall, known as the costophrenic angle.
The patient has a high body temperature, presenting with remittent fever. The white blood cell count is elevated, with neutrophils increasing to over 80%, and a left shift in the nucleus.
Chest X-ray examination is the primary diagnostic method for empyema. Free pleural effusion initially accumulates at the base of the pleural cavity, usually between the lung base and the diaphragm, causing the lung tissue to float slightly upward. With a small amount of effusion, the costophrenic angle becomes blunted, typically around 200 ml. If the patient cannot undergo chest X-ray in a sitting or standing position for any reason, attention should be paid to comparing the density of both sides on a supine chest X-ray. The side with effusion generally shows increased density. Alternatively, a lateral decubitus view with the affected side down can be used, where a small amount of effusion can be seen along the lateral wall of the affected pleural cavity, presenting as a uniform, deepened shadow between the inner edge of the ribs and the outer edge of the lung.
With a moderate amount of effusion, the X-ray shows a dense, arc-shaped shadow in the lower chest, higher on the outside and lower on the inside, covering the entire diaphragmatic surface. The effusion volume is approximately 500–1000 ml.
With a large amount of effusion, the fluid can reach the lung apex, compressing and collapsing the lung tissue. The translucency of the affected side further decreases, the pleural volume increases, the intercostal spaces widen, the ribs flatten, the mediastinum shifts to the healthy side, and the diaphragm descends. On the left side, the contrast with air in the gastric bubble makes it easier to visualize, while on the right side, the similar densities of the liver and effusion make differentiation difficult.
When effusion is combined with atelectasis, changes in the mediastinum, diaphragm, and chest wall are often not obvious. The effusion shadow, higher on the outside and lower on the inside, also varies with the location of atelectasis and is often atypical.
When combined with pyopneumothorax or bronchopleural fistula, an air-fluid level may be seen (Figure 1).
Localized empyema is often found on the posterior and lateral walls of the pleural cavity. The X-ray may show a localized area of increased density, with the central part denser and the periphery gradually lighter. On a tangential view, it appears as a uniform, localized shadow attached to the chest wall, with a broad base, clear inner edge, and a flat or semicircular protrusion into the lung field. It may also present as interlobar effusion, subpulmonary effusion, or mediastinal effusion (Figure 2), often requiring differentiation from pleural lesions, lung tumors, subphrenic abscess, or liver abscess.
Interlobar effusion refers to pleural effusion located within the interlobar fissure. It can only be visualized on X-ray when the direction of the X-ray aligns with the interlobar fissure, typically showing clear edges, uniform density, and a spindle shape with elongated ends. The long axis of the shadow aligns with the direction of the interlobar fissure. With a large amount of effusion, it may appear spherical.
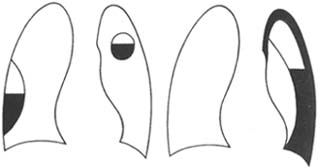
Figure 1 Hydropneumothorax
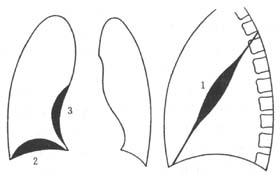
Figure 2 Encapsulated effusion
Subpulmonary effusion on X-ray shows the highest point of the diaphragmatic dome shifting outward on the posteroanterior view and backward on the lateral view, or the diaphragmatic shadow appears thickened. When a shadow resembling an elevated diaphragm is observed, subpulmonary effusion should be suspected. Using a supine or lateral decubitus view with the affected side down, the fluid drains away from the diaphragm, revealing the true position of the diaphragm.
CT examination: Empyema appears as an arc-shaped, uniform dense shadow parallel to the chest wall. Changing the patient's position can determine if the effusion is mobile. A large effusion entering the pulmonary fissure can displace the lower lung inward and backward. A large effusion adjacent to the posterior edge of the right liver lobe on CT scan shows a blurred posterior edge of the right liver lobe, making the boundary indistinct. This is a characteristic change of pleural effusion, known as the "interface sign."
Ultrasound: In the early stage when there is no cellulose deposition forming thickened pleura, there are no sediments in the fluid, and the hypoechoic area is clear without any echogenic spots. When there is a large amount of effusion, the lung tissue is compressed, and the gas in the lungs is absorbed. Ultrasound can reveal a triangular dense shadow within a large hypoechoic area, which moves with respiration. When the probe is near the diaphragm, a curved echogenic band representing the diaphragm can be seen, forming a wedge-shaped angle with the chest wall, known as the costophrenic angle.
The final diagnosis can be confirmed by thoracentesis to aspirate pus. The appearance, consistency, color, and odor of the pus can provide some clues to identify the type of pathogenic bacteria. Bacterial culture and drug sensitivity tests help in selecting effective antibiotics.
bubble_chart Treatment Measures
The treatment principles for acute empyema include three main aspects: systemic therapy, anti-infection, and pus drainage.
(1) Systemic Therapy
Encourage patients to eat and drink, paying attention to electrolyte supplementation, and consume a high-calorie, high-vitamin, high-protein diet. For critically ill or constitutionally weak patients, intravenous fluid replacement should be administered. If necessary, intravenous nutrition, plasma, albumin, or small, frequent transfusions of fresh blood should be given to correct anemia, enhance resistance, and promote early recovery.
(2) Anti-infection
Perform thoracentesis as early as possible to extract pus for bacterial culture and drug sensitivity testing. Select sensitive and effective antibiotics to control the condition promptly.
(3) Pus Drainage
1. Thoracentesis: In the early stages of some acute empyema cases, the pus is thin and can be easily extracted via thoracentesis. As long as the puncture site is well-chosen, the procedure can be successful. The physician performing the puncture should personally conduct a chest X-ray to understand the extent of the empyema and determine the puncture site under fluoroscopy. For localized empyema, puncture should first be performed at the site with the largest diameter of the pus cavity. For total empyema, the puncture is usually performed at the 7th intercostal space along the posterior axillary line. During the procedure, the patient should be positioned comfortably, typically in a semi-sitting position or sitting at a small table with arms resting on the table to avoid excessive fatigue and facilitate the puncture. Local anesthesia with 2% procaine or lidocaine is used. A large needle (18–22 gauge) with a length of at least 5 cm should be selected; otherwise, it may be difficult to penetrate the chest wall. The needle should be inserted along the upper edge of the rib to avoid injuring the intercostal nerves and blood vessels. The needle tip should generally point posteriorly and superiorly, so that after entering the pleural cavity, it remains close to the chest wall, minimizing the risk of injuring lung tissue. Before aspirating a large amount of fluid, the needle can be advanced an additional 0.5–1 cm, with the bevel facing the chest wall. This prevents the needle tip from slipping out of the pleural cavity during the procedure and avoids blockage by expanding lung tissue, ensuring complete fluid drainage. During each thoracentesis, every effort should be made to completely aspirate the pus, and after drainage, an appropriate amount of sensitive antibiotics should be injected into the pleural cavity through the puncture needle. Some cases of empyema can be cured with repeated thoracentesis and systemic therapy. If the pus is too viscous to be aspirated due to the causative bacteria, pleural lavage can be performed during the puncture. After aspirating some pus, inject an equal volume of saline or 2% sodium bicarbonate solution along with fibrinolytic agents (e.g., trypsin), and repeat the lavage until the aspirate becomes clear. Note that the volume of lavage fluid injected each time should not exceed the total volume of fluid aspirated, to avoid increasing intrapleural pressure and spreading pus to other areas, leading to infection dissemination. The reason thoracentesis may not completely cure empyema is that as the condition improves, the pus cavity shrinks, making puncture localization increasingly difficult, and residual pus cavities may persist.
2. Closed Thoracic Drainage: Closed thoracic drainage is required for acute empyema with rapid onset, large and viscous effusions, critical condition, or toxic symptoms, especially when effusions rapidly reaccumulate after thoracentesis. It is also necessary for pyopneumothorax complicated by bronchopleural or esophagopleural fistula.
Closed thoracic drainage can be performed using the trocar catheter insertion method (Figure 3). Under local anesthesia, a 0.5 cm skin incision is made, and the trocar is inserted through the intercostal space into the pleural cavity. The metal stylet is withdrawn, and a drainage tube is inserted through the outer sheath. The outer sheath is then removed, and the tube is secured to the skin and connected to a drainage bottle. This method is simple but has limitations: the drainage tube is usually narrow due to the outer sheath, leading to inadequate drainage, which may not meet the needs of empyema treatment. Additionally, withdrawing the outer sheath may contaminate the area around the drainage tube, causing infection and reducing or eliminating the seal around the tube, thereby affecting lung re-expansion.
Intercostal incision and tube insertion drainage (Figure 4): After local anesthesia, make a skin incision of about 2 cm, bluntly dissect each layer of muscle with hemostatic forceps until reaching the pleural cavity. Then, use curved hemostatic forceps to grasp the front end of the drainage tube and insert it directly into the pleural cavity. This method allows for the insertion of a thicker drainage tube, but the procedure is more complex and requires certain anatomical knowledge and experience.
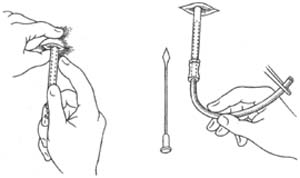
Figure 3 Catheter puncture and cannulation method
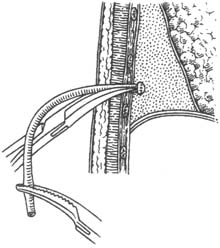
Figure 4 Intercostal incision and tube insertion
In recent years, various types of specialized closed thoracic drainage tubes have been widely used (Figure 5). This method involves making a skin incision of about 1 cm under local anesthesia, then directly inserting the specialized drainage tube into the thoracic cavity. Once the desired depth is reached, the needle core is withdrawn, and the tube is fixed and connected to the drainage bottle, completing the closed thoracic drainage procedure. This method is convenient and quick, with no contamination around the drainage tube. The thickness of the drainage tube can be selected as needed, offering outstanding advantages. As a result, it is widely applied with satisfactory outcomes.
3. Interventional Therapy Loculated empyema often occurs in the paravertebral groove. Due to its location, placing a closed thoracic drainage tube is inconvenient. If a drainage tube is placed on the back, the patient cannot lie flat, severely affecting rest, which is difficult for patients to accept. In recent years, the author has adopted the vascular puncture and catheterization method to perform catheter drainage and irrigation of the abscess cavity, achieving satisfactory results (Figure 6).
Figure 5 Specialized closed thoracic drainage tube

Figure 6 Interventional catheter irrigation
After local anesthesia with 2% procaine or lidocaine, a venous puncture needle is inserted into the abscess cavity, and pus is aspirated to confirm the needle tip is indeed within the cavity. A metal guidewire is then inserted, and the venous puncture needle is withdrawn. A pigtail-shaped catheter used for heart blood vessel angiography is advanced along the guidewire. Pus is aspirated through the catheter, followed by repeated irrigation. Antibiotics and fibrinolytic drugs can also be injected. The advantages of this method are: ① The catheter is thin and soft, causing minimal patient discomfort and not affecting the ability to lie flat; ② The catheter tip is pigtail-shaped, preventing tissue injury, allowing confident advancement to break fibrin septa within the abscess cavity, turning it into a single cavity for easier drainage; ③ The catheter is radiopaque, facilitating fluoroscopic observation of the abscess cavity's size; ④ As the abscess cavity shrinks during the healing process, the catheter can be gradually withdrawn, but as long as pus can still be aspirated, it confirms the catheter remains within the cavity, overcoming the difficulty of repeatedly locating the cavity during thoracentesis; ⑤ The catheter is thin, eliminating the need for dressing changes upon removal after empyema healing. This method has many advantages and proven efficacy, and is expected to be widely used in the future.




