| disease | Acute Angle-closure Glaucoma |
| alias | Congestive Glaucoma |
Angle-closure glaucoma is more common in women, with a male-to-female ratio of 1:3, typically occurring in individuals over 40 years old. The highest incidence is observed between the ages of 50 and 70. It often affects both eyes sequentially, usually within 5 years, though in rare cases, the interval may exceed 10 years. During an attack, hyperemia in the anterior segment of the eye is frequently observed, which is why it was previously referred to as congestive glaucoma. However, hyperemia is not the fundamental characteristic of this condition, and some individuals may not exhibit hyperemia during an attack.
bubble_chart Etiology
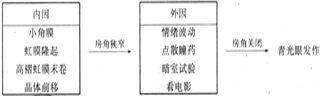
1. Internal factors: Anatomical and physiological factors.
(1) Variations within the normal range of anatomical structure and genetic defects: such as microphthalmos, small angle membrane, hyperopia, shallow anterior chamber, and high-rolled red membrane edge, which result in a shallow anterior chamber and narrow angle, leading to impaired aqueous humor drainage.
(2) Physiological changes: Pupillary block, shallow anterior chamber, and narrow angle, with grade II pupil dilation being a key condition. Additionally, with advancing age, the lens thickens over time, gradually pressing against the pupillary margin, creating pupillary block between the iris and lens. This causes the posterior chamber pressure to exceed the anterior chamber pressure. Coupled with weakened elasticity of the corneal-scleral membrane, which lacks the ability to compensate for sudden pressure increases, the peripheral iris is pushed forward, resulting in iris bombe and angle closure, thereby increasing intraocular pressure.
2. External factors
(1) Emotional hormones: Dysfunction of the central nervous system, imbalance of excitation and inhibition in the cerebral cortex, and impairment of the diencephalic intraocular pressure regulation center. Vascular motor nerve dysfunction causes congestion and edema of the pigment membrane, while sympathetic nerve excitation leads to pupil dilation, both of which can push the iris root toward the periphery, obstructing the angle.(2) The use of mydriatic drops, dark room testing, or prolonged exposure to movies or television can cause pupil dilation, angle obstruction, and subsequent elevation of intraocular pressure.
Schematic diagram of angle-closure glaucoma onset:
bubble_chart Pathological Changes
An increase in intraocular pressure can lead to a series of pathological tissue changes in the eye.
1. Acute stage: Manifested as circulatory disturbances and tissue edema in the eye, corneal edema, congestion and edema of the iris and ciliary body, and even exudation, dilation of blood vessels in the bulbar conjunctiva, and dilation, congestion, or even hemorrhage of retinal blood vessels.
In the initial stage of acute angle-closure glaucoma, the iris stroma is highly congested and edematous, and the iris root shifts forward to closely contact the trabecular meshwork, making the anterior chamber angle narrower or completely occluded. During this period, the anterior chamber angle is merely in contact without fibrous adhesion, and the acute symptoms can resolve after the acute phase. If improperly treated or recurrent, prolonged contact between the iris root and the trabecular meshwork can lead to fibrosis and degeneration of the iris stroma and the trabecular meshwork, resulting in permanent adhesion. The occluded anterior chamber will not reopen, and Schlemm's canal may become deformed due to compression, permanently losing its aqueous humor drainage function.
Primary glaucoma often affects both eyes, may occur sequentially, and has a familial hereditary predisposition, being genetically related.
bubble_chart Clinical Manifestations
The clinical manifestations of acute angle-closure glaucoma are complex. Due to its sudden onset, severe condition, obvious angle closure, varied symptomatic presentations, and differing durations of the disease process, there is significant congestion in the anterior chamber when the angle closes. Its clinical manifestations are divided into six stages.
1. **Clinical Pre-stage**: Since angle-closure glaucoma mostly affects both eyes and attacks occur sequentially, if one eye has already experienced an attack or has a history of attacks, the other eye, though not yet attacked, already exhibits anatomical conditions such as a shallow anterior chamber and narrow angle. It is likely to experience an attack sooner or later, and under certain triggering conditions or provocative tests, intraocular pressure may suddenly rise. Therefore, this eye is referred to as the clinical pre-stage and requires close monitoring. If possible, performing a prophylactic peripheral iridectomy on such eyes can achieve permanent therapeutic effects.
2. **Prodromal Stage**: Some patients experience multiple minor attacks before an acute episode, presenting with transient rainbow vision (halos), blurred vision, or eye distension. However, patients often mistake these symptoms for a common cold or fatigue rather than an eye disease. Detailed examination may reveal slightly elevated intraocular pressure and mild congestion in the anterior segment. These symptoms usually resolve completely after sleep or adequate rest, returning to normal. Prophylactic peripheral iridectomy on such eyes can prevent acute intraocular pressure elevation, addressing the issue before it arises.
⑴ **Symptoms**: The onset is sudden, with most or all of the angle closed, leading to a sharp rise in intraocular pressure. Subjective symptoms include severe eye pain, even nausea, vomiting, elevated body temperature, and significant visual impairment. In the initial stage, patients may see rainbow halos around lights, followed by a rapid decline in vision. In severe cases, vision may be reduced to counting fingers or even only light perception. The reasons for vision loss are twofold: first, due to corneal edema, and second, due to generalized ischemia of the optic nerve caused by high intraocular pressure. If intraocular pressure is promptly controlled, vision can improve and recover to a useful level. However, if high intraocular pressure persists unchecked, ischemic atrophy of the optic disc may occur, leading to blindness.
**Nausea, vomiting, bradycardia, and sweating**: These symptoms result from an oculovagal reflex and are often misdiagnosed as gastrointestinal disorders, delaying timely treatment of the condition.
⑵ **Signs**
① **Static blood in the anterior segment**: This occurs due to secondary venous congestion following sustained high intraocular pressure, leading to dilation of conjunctival and iris vessels.
② **Corneal edema**: Under high intraocular pressure, aqueous humor infiltrates the corneal stroma through damaged endothelial cells, resulting in a hazy appearance and endothelial folds. In the stage of attack, many patients exhibit dusty pigmentary KP (keratic precipitates) on the corneal endothelium. Over time, aqueous humor may infiltrate beneath the corneal epithelium, causing bullous corneal degeneration.
③ **Shallow anterior chamber and aqueous flare**: After venous congestion in the anterior segment, proteins leak into the aqueous humor, causing mild turbidity. In advanced stages, free pigment may be present in the aqueous humor, giving it a shimmering appearance. This can be misdiagnosed as iridocyclitis.
④ **Pupil dilation and pupillary block**: Persistent high intraocular pressure paralyzes and partially atrophies the pupillary sphincter. Due to heavier adhesions in the superior and inferior angles, the pupil often dilates to grade II, assuming a vertically oval shape. As the pupil dilates further, the iris moves closer to the anterior lens capsule, obstructing the flow of aqueous humor from the posterior to the anterior chamber, resulting in relative pupillary block. If aqueous humor cannot enter the anterior chamber at all, it is termed absolute pupillary block. In milder cases, the pupil may return to normal after intraocular pressure is reduced. However, if peripheral anterior synechiae of the iris are present, the pupil may remain permanently dilated.
⑤Iris membrane swelling, crypt disappearance: Posterior synechia of the iris pupillary part and peripheral anterior synechia of the iris. Posterior synechia of the iris occurs due to iris congestion and protein exudation, which leads to extensive contact with the anterior capsule of the lens, making adhesion highly likely. Severe congestion, elevated posterior chamber pressure, significant fibrous exudation, iris edema, and corneal epithelial edema promote peripheral iris bulging or the formation of anterior synechia of the iris, resulting in anterior chamber angle blockage. If peripheral iris adhesion has not occurred and only bulging is present, the occluded angle may reopen after intraocular pressure decreases. However, if adhesion has already formed, the angle will not reopen even if intraocular pressure drops. Due to the sudden rise in intraocular pressure exceeding the blood pressure of the iris microcirculation, blood vessels become occluded, leading to segmental iris atrophy (also called sectoral atrophy) or even necrosis. Some cases may present as diffuse atrophy, which is particularly noticeable at the pupillary margin.
⑥Formation of glaucomatous subcapsular flecks: A sharp and persistent increase in intraocular pressure causes nutritional disturbances in the lens, leading to the appearance of semi-translucent grayish-white or milky-white opacities in the subcapsular region of the lens within the pupillary area. These are known as glaucomatous subcapsular flecks. Such flecks are observed in one-third of patients during the stage of attack. In the early stages, the opacities appear as patches, which may partially resolve and become transparent again as intraocular pressure decreases. They may also present as dots, lines, or semicircular shapes, with milder cases showing only a few scattered small dots. After the acute symptoms subside, no new flecks form. The triad of posterior corneal pigment (KP), segmental atrophy of the iris, and glaucomatous subcapsular flecks is referred to as the "triad of acute angle-closure glaucoma." Since these signs persist for years after an attack, they hold significant diagnostic value for retrospective diagnosis.
⑶Changes in intraocular pressure and tonography: During the acute stage of attack, intraocular pressure rises abruptly, generally exceeding 5.32 kPa (40 mmHg), with some cases reaching over 10.64 kPa (80 mmHg), resulting in an explosive pattern. In milder cases, the rise in intraocular pressure causes pupillary dilation, relieving pupillary block, and the pressure may decrease without treatment or return to normal after rest or sleep. One or two days after an acute attack, even if the angle remains closed and the pupil does not return to normal, intraocular pressure may normalize or even become hypotonic. This is not necessarily due to damage to the ciliary body's secretory function caused by high intraocular pressure but is termed "ciliary body shock." Medication must not be discontinued at this stage to prevent a "rebound" in intraocular pressure.
During an acute attack, before angle closure occurs, the angle remains a normal narrow angle, and trabecular function is unimpaired. The C-value is normal. However, once angle closure occurs, the C-value may drop to zero. After the acute attack subsides and the angle fully reopens, the C-value can return to normal.
⑷Fundus changes: During an acute attack, the fundus is difficult to visualize. However, after instilling glycerin, examination may reveal optic disc hyperemia and edema, marked engorgement of the central retinal vein, and small dot-like or flame-shaped hemorrhages around the disc. After intraocular pressure decreases, the optic disc color becomes significantly paler, but no glaucomatous cupping or enlarged excavation is observed, instead presenting as ischemic optic neuropathy.
4. Remission stage: With drug treatment or timely intervention, acute angle-closure glaucoma may resolve naturally, with intraocular pressure returning to normal, conjunctival congestion disappearing, the cornea clearing, and vision recovering to pre-attack levels. If there is no extensive peripheral anterior synechiae, the C-value may remain within the normal range, and the angle may reopen. Some cases may exhibit partial peripheral anterior synechiae. The remission stage is temporary; if peripheral iridectomy is not performed, acute attacks may recur, and intraocular pressure may rise again, eventually leading to blindness. The duration of remission varies, ranging from 1–2 years to as short as 1–2 months, with some cases recurring daily. Performing a peripheral iridectomy during this stage can prevent recurrence.
During the remission stage, intraocular pressure is normal, the angle is open, and the C-value is normal, but the facility of outflow remains unimproved. This decrease in intraocular pressure is generally temporary, and pressure may rise again, leading to another attack. Even if a peripheral iridectomy has been performed, it may not achieve the therapeutic goal.
5. Chronic progressive stage: If acute angle-closure glaucoma is not treated promptly and appropriately during the acute stage of attack, symptoms may not fully resolve, transitioning into a chronic progressive stage. This stage is characterized by grade II elevation of intraocular pressure, partial clearing of the cornea, grade I pupillary dilation, varying degrees of peripheral anterior synechiae in the angle, and minimal early fundus changes. In advanced stages, it resembles chronic angle-closure glaucoma, manifesting as glaucomatous cupping of the optic disc and visual field defects, with gradual progression of the disease.
6. Absolute stage: The absolute stage of acute angle-closure glaucoma manifests as high intraocular pressure, complete loss of vision, pupils often larger than 6-7mm, the anterior chamber angle showing extensive peripheral iris anterior synechiae, or even complete occlusion, with the iris exhibiting segmental atrophy and diffuse pigment shedding.
Under high intraocular pressure, the supply of the iris membrane stirred pulse may lead to local circulatory disturbances, ischemia, and even segmental atrophy of the iris membrane stroma, or generalized atrophy. The atrophied iris membrane may exhibit attached dust-like pigment granules or pigment shedding.
1. Preclinical stage: The fellow eye of an eye with a typical history of attacks; although it has never had an attack, if the anterior chamber depth is ≤1/4 CK, the angle narrowness is grade III (III), and the dark room and prone tests are positive, it is considered the preclinical stage.
2. Stage of attack: During an acute attack, the diagnosis is not difficult if typical manifestations are present. However, if the manifestations are atypical and the examination is not thorough, the acute attack of glaucoma is often misdiagnosed as iridocyclitis or, due to severe headache, nausea, and vomiting, the ocular examination is neglected, leading to misdiagnosis as an internal disease (see Table 17-2-10).
Table 17-2-1 Differential Diagnosis of Acute Angle-Closure Glaucoma, Acute Iridocyclitis, and Acute Conjunctivitis
| Acute Angle-Closure Glaucoma | Acute Iridocyclitis | Acute Conjunctivitis | |
| Vision | Severely decreased | Varying degrees of decline | Normal |
| Symptoms | Severe eye pain, headache, nausea, vomiting | Photophobia, tearing, deep eye and orbital pain, tenderness in the ciliary region | Foreign body sensation, burning sensation, mucopurulent discharge |
| Congestion | Static blood in the anterior eye | Mixed congestion | Conjunctival congestion |
| Cornea | Hazy opacity | Grade I edema or none | Clear |
| KP | Dust-like pigmented | Pigmented KP of varying sizes | None |
| Anterior chamber | Shallow, aqueous flare | Marked aqueous flare | Normal |
| Pupil | Vertically oval dilation, sluggish light reflex, no posterior synechiae | Constricted, sluggish light reflex, no posterior synechiae | Normal |
| Lens | Some may have glaucomatous flecks | Exudates on the anterior capsule | Normal |
| Angle | Closed | Open or closed | Normal |
| Intraocular pressure | Significantly elevated | Mostly normal or slightly elevated | Normal |
The diagnosis of the acute stage of attack is mainly based on the following criteria:
(1) Precipitating psychological or emotional factors, accompanied by sudden severe eye distension pain, reflex headache, nausea, vomiting, etc.
(2) Rapid decline in vision, even to light perception.
(3) Sudden elevation of intraocular pressure, with the eye feeling hard as stone, and the pressure may exceed 10.64 kPa (80 mmHg).
⑷There is obvious congestion in the anterior segment, and in severe cases, it may be accompanied by conjunctival and eyelid edema.
⑸ The cornea appears steamy with edema, losing its normal transparency and smooth surface, and sensation is diminished or blunted.
⑹ The pupil is often vertically oval and dilated, with a greenish reflection visible in the pupillary area.
3. Intermittent stage: Diagnosis during this phase is crucial. Although intraocular pressure (IOP) is normal and there is no local congestion, the glaucoma is not cured and may recur. Key diagnostic points include:
⑴ A detailed history of acute episodes, often with 1-2 typical attacks, followed by a decrease in IOP and symptom relief after treatment or rest.
⑵ The presence of dust-like pigmented KP (keratic precipitates) on the posterior cornea and pigment on the anterior lens capsule. The cornea exhibits grade I edema.
⑶ Sluggish pupillary light reflex.
⑷ After an acute attack, the anterior chamber angle often shows residual peripheral anterior synechiae of the iris and pigment deposits. Some or all components of the glaucomatous triad may be present.
⑸ Repeated IOP testing may sometimes show normal IOP and normal C-value.
4. Chronic progressive stage:
⑴ Transitioning from the acute attack stage, IOP remains persistently elevated at grade II, with relatively mild subjective symptoms.
⑵ The pupil is larger, and most of the anterior chamber angle shows permanent synechiae.
⑶ Glaucomatous cupping appears in the fundus, with visual field defects.
⑷ The glaucomatous triad is present.
5. Absolute stage:
⑴ After an acute attack, vision is completely lost, IOP remains persistently elevated, the pupil is vertically oval and larger than 6-7 mm, sometimes with grade I ciliary congestion. A series of degenerative changes occur in ocular tissues.
⑵ The glaucomatous triad is pronounced, and the optic nerve is completely atrophied.
⑶ The cornea develops bullous keratopathy, and neovascularization of the iris occurs.
bubble_chart Treatment Measures
Glaucoma is a multifactorial disease involving both the eyes and the entire body. The goal of treatment is to relieve or reduce the resistance to aqueous humor outflow, suppress aqueous humor production, decrease intraocular volume, and improve blood supply to the optic nerve. Therefore, treatment should be comprehensive, combining medication and surgery, as well as systemic and local approaches.
During the treatment of glaucoma, the hypotensive effects of Chinese medicinals vary in terms of administration routes and mechanisms. Some primarily facilitate the drainage pathway of aqueous humor, while others reduce volume or decrease aqueous humor production, and some combine all three. To enhance efficacy, medications should be rationally selected based on the different types and stages of glaucoma.
Principles of Glaucoma Treatment
Pharmacological Treatment
1. Acute Angle-Closure Glaucoma
(1) Acute attack stage: Once diagnosed, systemic and local medications should be administered immediately. First, use miotics, oral carbonic anhydrase inhibitors, and intravenous hyperosmotic agents.
① Application of miotics: To prevent peripheral anterior synechia of the iris and reopen the angle as quickly as possible. Strong miotics should be avoided during the acute phase, as they may increase the chance of iris-lens contact, worsen pupillary block, and provoke uveal inflammatory reactions.
Commonly used miotics
Pilocarpine: Its mechanism of action is to open the occluded anterior chamber angle, improve aqueous humor circulation, and reduce intraocular pressure. In the early stage, a 1–2% solution is typically used, administered every 3–5 minutes for 3–5 doses. Once the pupil constricts and intraocular pressure decreases, switch to every 1–2 hours or 3–4 times daily. If systemic hypotensive agents are used first, the miotic effect is better. After each instillation, compress the lacrimal sac and keep the eyes closed for a few minutes.
Physostigmine (eserine): It has a strong miotic effect but is irritating and should not be used long-term. Frequent instillation can cause systemic toxicity, worsen local congestion, and lead to risks such as elevated intraocular pressure. During the acute phase, instill 1–2 times in the first half-hour, then switch to pilocarpine for better efficacy. However, the two should not be used simultaneously due to the risk of toxicity and counteracting miotic effects.
② Application of carbonic anhydrase inhibitors: Carbonic anhydrase catalyzes the conversion of CO2 and water into carbonic acid in tissues, which then dissociates into H+ and HCO-3. In the posterior chamber, HCO-3 (bicarbonate ion) combines with N-a to form sodium bicarbonate, increasing osmotic pressure in the posterior chamber, raising aqueous humor production, and elevating intraocular pressure.
Carbonic anhydrase inhibitors can suppress the activity of carbonic anhydrase, reducing aqueous humor production. Commonly used carbonic anhydrase inhibitors include:
Acetazolamide (Diamox): A sulfonamide derivative that inhibits carbonic anhydrase. By suppressing carbonic anhydrase in the ciliary epithelium, the sodium bicarbonate content in aqueous humor decreases, lowering osmotic pressure and reducing aqueous humor formation, thereby decreasing intraocular pressure.
Peak plasma concentration is reached 2 hours after oral administration, with effects lasting 8–12 hours. Typically, dosing every 6 hours is required. For acute angle-closure glaucoma, the dose can be increased: an initial 0.5g, followed by 0.25g every 6 hours. Concurrent use of an equivalent amount of sodium bicarbonate to alkalinize the urine facilitates excretion.
Long-term use increases potassium excretion in urine, potentially disrupting systemic electrolyte balance. Therefore, oral potassium supplements, such as 1g potassium chloride-coated tablets or 10ml of 10% KCl solution, should be given 2–3 times daily.
Dichlorphenamide (Daranide): A potent carbonic anhydrase inhibitor. Effects begin 1 hour after oral administration, with hypotensive effects lasting 6–12 hours. Dosage is 50mg orally, 1–3 times daily.
③Application of hyperosmotic agents: Any substance that can improve the osmotic pressure gradient between blood and aqueous humor, increase blood osmotic pressure, thereby absorbing intraocular water and reducing intraocular pressure can be called a hyperosmotic agent. It is usually administered intravenously.
The hypotensive mechanism of hyperosmotic agents has two aspects: primary direct osmotic effect and secondary indirect osmotic effect. The osmotic agent is preferably a macromolecular compound, making it difficult to pass through the blood-aqueous barrier. This maintains a certain osmotic pressure gradient between the blood and aqueous humor, facilitating the movement of water from the tissues.
Currently, commonly used hyperosmotic agents in clinical practice include urea and mannitol.
Mannitol: After entering the bloodstream, it increases plasma osmotic pressure, creating an osmotic gradient between the blood and aqueous humor. This causes water to move from the tissues into the blood, increases aqueous humor outflow, reduces intraocular water content, and thereby lowers intraocular pressure. A 20% solution is administered intravenously for patients in the acute stage of glaucoma attack or before various types of glaucoma surgery.
Urea: After a large dose of urea is administered, urea levels in the blood rise rapidly and distribute throughout the body fluids. However, due to the blood-aqueous barrier, urea diffuses into the aqueous humor very slowly. Thus, after urea administration, its concentration in the blood is much higher than in the aqueous humor, creating an osmotic pressure gradient (i.e., a difference) between the blood and aqueous humor. Water then moves from the eye into the blood, increasing aqueous humor outflow and dehydrating the vitreous, reducing its volume and lowering intraocular pressure.
Urea is preferably administered intravenously at a dose of 1–0.5 g per kilogram of body weight, dissolved in 10% invert sugar to form a 30% urea solution, and infused at a rate of 60 drops per minute. It can be given once daily. Alternatively, a 30% urea and 10% mannitol solution can be prepared for intravenous injection. Urea can also be taken orally, but its effect is slower and may easily cause nausea and vomiting. A 50% glucose solution administered intravenously also has some intraocular pressure-lowering effect, though its efficacy is relatively poor.
④Use of sedatives: Oral or intramuscular sedatives or retrobulbar block can alleviate patient anxiety and fear, relieve pain and symptoms, help reduce intraocular pressure, and promote recovery. Commonly used analgesics include Luminal, Wintermin, and Indomethacin.
Indomethacin: It inhibits prostaglandin synthesis, thereby suppressing the increase in aqueous humor prostaglandin levels caused by traction or trauma to ocular tissues, which can lead to lowered intraocular pressure and pupil constriction. Thus, indomethacin has a significant effect on lowering intraocular pressure.
2% lidocaine (with a small amount of adrenaline) 3–4 ml, injected retrobulbarly or temporally, can relieve pain, reduce pressure, alleviate symptoms, and accelerate treatment efficacy.
⑵Intermittent stage: After emergency treatment, if the anterior chamber angle reopens entirely or over 2/3 of its circumference, and both intraocular pressure and C-value return to normal, peripheral iridectomy can be performed.
⑶Chronic stage: If the anterior chamber angle has developed organic adhesions, with a low C-value and high intraocular pressure, filtering surgery can be performed once ocular vascular reactivity has subsided. Preoperative medication should be used to lower intraocular pressure to a relatively low level to reduce postoperative complications.
⑷Absolute stage: To relieve pain, filtering surgery, combined anti-glaucoma surgery, or cyclocryotherapy or cyclodiathermy may be performed.
Surgical treatment
Surgical treatment of glaucoma is one of the effective measures for managing glaucoma. The mechanisms of surgery include: first, restoring and enhancing the aqueous humor drainage pathway to ensure smooth outflow, and second, reducing the production of aqueous humor to prevent an increase in intraocular pressure. There are many traditional surgical methods, some of which remain widely used. For example, surgeries to prevent pupillary block, such as peripheral iridectomy, create a new channel to allow aqueous humor to contact the trabecular meshwork, preventing angle closure and controlling the occurrence of angle-closure glaucoma. Some surgeries are gradually being phased out, such as sclerostomy, trephination, and iridencleisis. These procedures aim to drain aqueous humor through surgical incisions to the outside of the eye, thereby reducing intraocular pressure. Currently, the most widely used anti-glaucoma surgeries in clinical practice are trabeculectomy, which unblocks the original aqueous humor drainage pathway, and Schlemm's canalotomy. Both procedures yield relatively good outcomes. Of course, there are many types of glaucoma surgeries, but none is universally applicable. The choice of surgical indication must be carefully and seriously based on the specific condition of the affected eye, with thorough preoperative preparation. The goal is to both maintain good visual function and restore normal intraocular pressure levels.
2. In the acute stage of glaucoma attack, surgery should not be rushed. Instead, drug treatment should be administered first to constrict the pupil, reduce intraocular pressure, alleviate edema of intraocular tissues, and mitigate hypertensive iris membrane reactions. Alternatively, if intraocular pressure still fails to decrease after 24 hours of drug treatment, surgery can then be performed. This approach not only enhances therapeutic efficacy but also reduces surgical complications. The role of surgery is not merely to address the issue of elevated intraocular pressure—lowering it to a normal level does not equate to a cure for glaucoma. Surgery cannot halt the dystrophic process of the optic disc in some fairly advanced stages of glaucoma but can only slow its progression. Therefore, postoperative drug treatment remains significant. The question of whether to perform surgery for advanced-stage glaucoma with tubular visual fields warrants serious consideration. Some argue that surgery carries the risk of sudden blindness and thus oppose it, advocating for drug treatment instead. However, current evidence suggests that as long as central vision remains relatively good, preoperative intraocular pressure is adequately controlled, adrenaline-like drugs are avoided during surgery, and sufficient rest is ensured, the likelihood of sudden blindness during surgery is minimal.






