| disease | Rheumatic Mitral Stenosis |
Rheumatic valvular heart disease is a chronic cardiac condition resulting from acute rheumatic fever affecting the heart, and it remains quite prevalent in our country. Mitral valve involvement is the most common in rheumatic valvular heart disease, followed by aortic valve involvement, while tricuspid valve involvement is rare, and pulmonary valve involvement is even rarer. Chronic rheumatic heart disease can affect multiple valves. Clinically, isolated mitral valve disease is the most common, accounting for about 70%, followed by combined mitral and aortic valve disease at approximately 25%. Isolated aortic valve disease accounts for about 2–3%, while tricuspid or pulmonary valve disease usually coexists with mitral or aortic valve disease.
bubble_chart Pathological Changes
Rheumatic fever mostly occurs during adolescence and is an allergic disease. The lesions affect the collagen fibers of connective tissue, causing mucoid degeneration and fibrinoid necrosis, gradually leading to fibroblast proliferation, and infiltration by lymphocytes and monocytes, forming rheumatic bodies. As the disease progresses, rheumatic bodies become fibrotic and turn into scar tissue. The course of rheumatic fever develops relatively slowly, generally lasting 4 to 6 months, but often recurs, causing gradual worsening of tissue damage. Rheumatic fever often affects the heart, causing pancarditis, involving the pericardium, myocardium, and endocardium. The most severe damage caused by recurrent rheumatic fever is to the endocardium, particularly the endocardial tissue of the mitral valve. Long-term recurrent rheumatic inflammation, along with mechanical injury from turbulent blood flow and platelet aggregation, leads to mitral valve lesions, mainly including fusion of the valve commissures, fibrous thickening of the valve leaflets, fibrosis and shortening of the chordae tendineae and/or papillary muscles, fusion, and calcification of the valve leaflets. The above pathological process generally takes 10 to 30 years, but commissural fusion and valve leaflet fibrosis sometimes require only 2 to 3 years. Fusion of the mitral valve commissures first occurs at the anterolateral and posteromedial commissures, then gradually extends toward the central part of the valve orifice. In grade I stenosis, the valve orifice diameter is about 1.3 cm; in grade II stenosis, it is 0.8 to 1.2 cm; and in grade III stenosis, it is less than 0.8 cm. The longer the range of commissural fusion, the more severe the valve orifice stenosis. In grade I stenosis cases, although the valve orifice is narrowed due to commissural fusion, the mobility of the valve leaflets remains good, and the valve membrane appears diaphragm-like. In grade III stenosis patients, the mitral valve orifice diameter is only a few millimeters, with both the anterior and posterior leaflets showing fibrous thickening, shortening, or even calcification. The lesions in the posterior leaflet are often more severe than those in the anterior leaflet. The mobility of the posterior leaflet is lost, while the anterior leaflet retains some mobility. When the chordae tendineae and papillary muscles also exhibit thickening, adhesion, and shortening, the valve leaflets are pulled into the left ventricle, restricting movement, and the mitral valve takes on a funnel-like shape. In addition to valve orifice stenosis, insufficiency is often present. In cases of mitral stenosis, the left atrium is often enlarged and hypertrophied, with blood stagnating in the left atrium, potentially leading to thrombus formation in the atrial appendage. Thrombosis is more common in cases of atrial fibrillation.
Due to obstruction of pulmonary circulation blood return, the lung tissue becomes chronically congested, leading to interstitial edema and fibrosis. Many macrophages containing hemosiderin (heart failure cells) may be present in the alveoli.In adults, the mitral valve orifice area during opening is approximately 4–6 cm², with an orifice length of about 3.5 cm, allowing the passage of 2–3 fingers. When the commissures fuse and the orifice narrows by more than 50%, i.e., the orifice area becomes less than 2.5–3.0 cm², the flow of blood from the left atrium into the left ventricle through the mitral orifice begins to encounter obstruction. When the orifice is less than 2–2.5 cm², the left atrial pressure starts to rise to 2–2.7 kPa (15–20 mmHg), and the increased pulmonary circulation volume begins to manifest as dyspnea upon exertion. If the orifice further narrows to below 1.5 cm², the left atrial pressure may rise to 3.3–4 kPa (25–30 mmHg), and fluid from the pulmonary capillaries begins to enter the alveoli, leading to the appearance of moist rales at the lung bases. As left atrial pressure increases, the left atrium gradually hypertrophies, often resulting in atrial fibrillation. If the ventricular rate increases due to exertion or emotional stress, the patient may suddenly experience dyspnea, orthopnea, or even acute pulmonary edema. Concurrently, due to reduced cardiac output, systemic blood pressure decreases accordingly, causing the patient to feel dizzy, weak, and fatigued. In mitral stenosis patients with atrial fibrillation, blood stasis in the left atrium may lead to thrombus formation in the atrial appendage, and thrombus detachment can cause systemic embolism. Prolonged elevation of left atrial and pulmonary circulation pressures initially causes pulmonary arterioles to enter a spastic state, followed by thickening of the vessel walls and narrowing of the lumen, leading to obstructive pulmonary vascular disease. Simultaneously, the thickening of the interface between the pulmonary capillary membrane and alveolar membrane reduces the incidence of pulmonary edema caused by fluid leakage into the alveoli. In long-term grade III mitral stenosis cases, pulmonary arterial pressure gradually rises, with severe cases reaching 12–16 kPa (90–120 mmHg). Chronic pulmonary hypertension promotes right ventricular hypertrophy, eventually leading to right heart failure, manifested as jugular vein distension, hepatomegaly, and lower limb edema. Enlargement of the right atrioventricular ring may also result in functional tricuspid regurgitation.
bubble_chart Clinical Manifestations
Most patients with mitral stenosis seek medical attention around the age of 30. As the mitral valve lesion progressively worsens, left ventricular function is also impaired. Within 10 to 15 years of onset, cardiac function often deteriorates to grade 3–4. Although medical treatment can alleviate symptoms of heart failure, it cannot relieve the obstruction of the mitral valve and pulmonary vascular {|###|} sexually transmitted disease changes. Most untreated patients die around the age of 50 from pulmonary {|###|} stirred pulse hypertension, heart failure, atrial fibrillation, systemic embolism, or infective endocard {|###|} membrane itis.
Among cases of {|###|} wind-dampness-related mitral stenosis, only about 50% have a history of {|###|} wind-dampness fever or migratory polyarthritis. Generally, symptoms of mitral stenosis appear at least 10 years after {|###|} wind-dampness fever, with most cases manifesting after the age of 20. The clinical progression of mitral stenosis is slow. The initial stage [first stage] symptoms include dyspnea caused by pulmonary congestion due to valve orifice stenosis. Initially, shortness of breath occurs after heavy physical exertion, followed by its appearance after moderate or grade I exertion. Orthopnea, paroxysmal nocturnal dyspnea, and pulmonary {|###|} edema may occur during physical exertion, respiratory infections, emotional stress, or atrial fibrillation. {|###|} Cough is also a common symptom, occurring more frequently after exertion, during sleep, or during bronchitis episodes, with sputum appearing as white mucus. Some cases present with asthma-like attacks, palpitation, paroxysmal atrial fibrillation, lack of strength, fatigue, dizziness, and other symptoms. Patients may experience recurrent {|###|} hemoptysis, with varying amounts of bleeding. Bronchial mucosal {|###|} membrane bleeding may cause blood-streaked sputum, acute pulmonary {|###|} edema bleeding may present as pink frothy mucus, and rupture of varicose bronchial veins can lead to massive {|###|} hemoptysis. Advanced stage cases may exhibit symptoms of right heart failure such as hepatomegaly, ascites, and subcutaneous {|###|} edema. A minority of patients present primarily with systemic embolism as their first clinical symptom.
The pulmonary {|###|} stirred pulse second sound is accentuated and may show grade I splitting. In cases of pulmonary {|###|} stirred pulse hypertension with dilation of the pulmonary {|###|} stirred pulse and valve ring, a systolic ejection click may be heard after the first heart sound at the left second and third intercostal spaces, loudest during expiration and diminished or absent during inspiration. Occasionally, a soft, high-pitched blowing diastolic early intermediate stage [second stage] murmur (Graham-Steell murmur) due to relative pulmonary {|###|} stirred pulse regurgitation may be heard, intensifying at the end of inspiration and weakening during expiration. Cases with tricuspid regurgitation may exhibit a systolic murmur at the left fourth and fifth intercostal spaces, louder during inspiration and diminished during expiration or Valsalva maneuver. Patients with atrial fibrillation exhibit irregular heart rhythms. Right heart failure cases may show basal lung crackles, hepatomegaly, lower limb {|###|} edema, and sometimes signs of ascites. Cases complicated by embolism may present with central nervous system symptoms or limb motor dysfunction.
bubble_chart Auxiliary Examination
Chest X-ray examination: In early cases, posteroanterior chest X-ray films may show no abnormal signs. In cases with significant stenosis of the valve orifice, the left atrium is enlarged, and a dense double shadow of overlapping left and right atria can be seen on the right side of the cardiac shadow. The cardiac shadow is enlarged, with the left atrial appendage, right ventricle, and pulmonary artery expanding, while the aortic arch shrinks. The pulmonary artery cone is prominent, the branches of the pulmonary artery widen, and the hilar shadow deepens. The normal depression between the left ventricle and the aortic bulb disappears, and the left border of the cardiac shadow becomes straight. In cases with long-term pulmonary congestion, scattered spotty shadows due to hemosiderin deposition can be seen in the lung fields. Additionally, fine, short, horizontal lines with increased density (Kerley B lines) caused by chronic pulmonary lymphatic congestion may be observed in the lower lung fields. Barium meal X-ray examination in lateral or oblique views can reveal notches caused by the enlarged left atrium compressing the esophagus and displacing it posteriorly. The enlarged left atrium may also elevate the left main bronchus, increasing the angle between the two main bronchi. In cases of pure mitral stenosis, the left ventricle should not be enlarged; if the left ventricle is enlarged, concomitant mitral regurgitation should be highly suspected.
Electrocardiogram (ECG) examination: Grade I mitral stenosis cases may show no abnormalities on ECG. Left atrial hypertrophy presents as a widened and notched P wave and an enlarged biphasic P wave in the right precordial leads. Cases with pulmonary hypertension show signs of right axis deviation, right ventricular hypertrophy, and strain. Long-standing cases often exhibit atrial fibrillation.
Cardiac catheterization and angiography: Mitral stenosis cases do not routinely require cardiac catheterization, but for multivalvular diseases, cardiac catheterization and angiography help determine the presence and severity of other valvular lesions. Right heart catheterization can measure right ventricular, pulmonary artery, and pulmonary capillary wedge pressures, pulmonary vascular resistance, cardiac output index, and calculate the valve orifice area. In mitral stenosis, right ventricular, pulmonary artery, and pulmonary capillary wedge pressures are elevated, pulmonary vascular resistance increases, and cardiac output index decreases. Left heart catheterization can measure left atrial pressure and the mitral valve pressure gradient. In mitral stenosis, the mitral valve pressure gradient exceeds 0.7 kPa (5 mmHg). In early cases, the resting pressure gradient may be normal (0.3–0.4 kPa or 2–3 mmHg) but rapidly increases to over 1.3 kPa (10 mmHg) after exercise. Selective left ventriculography can assess the presence of mitral regurgitation and left ventricular systolic function. Aortic angiography can confirm the presence of aortic regurgitation.
Echocardiography: M-mode echocardiography shows left atrial and right ventricular enlargement. The mitral valve anterior leaflet exhibits a slow descent after the E peak during diastole, with reduced BE wave descent speed, presenting a "doming" pattern. Due to commissural fusion, the anterior and posterior leaflets move in the same direction (Figure 4). Cross-sectional echocardiography can reveal thickened valves with restricted mobility, irregular morphology, and a narrowed orifice, sometimes showing thickened and adhered subvalvular chordae. Echocardiography can also detect thrombi in the left atrial appendage or left atrium. Transesophageal echocardiography provides more reliable diagnosis of thrombi in the left atrial appendage and left atrium.
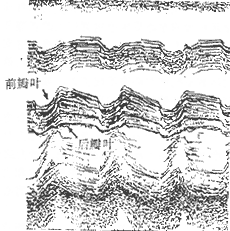
Figure 4. M-mode echocardiogram of mitral stenosis.
bubble_chart Treatment Measures
The effective treatment for mitral stenosis is surgical intervention to enlarge the narrowed valve orifice, relieve or reduce the mechanical obstruction of blood flow from the left atrium to the left ventricle, improve the hemodynamics of the cardiac and pulmonary circulation, or remove the severely damaged mitral valve and replace it with an artificial one. However, the surgery does not eliminate the disease cause of {|###|}wind-dampness infection, and atrial fibrillation persists in most patients postoperatively.
Surgical indications: Patients with mitral stenosis who exhibit clinical symptoms should be considered for surgical treatment. Patients with cardiac function class I may first undergo {|###|}Saposhnikovia Root dampness-heat episodes, appropriately limit physical activity, and maintain hygienic habits in daily life while delaying surgery. Patients with cardiac function class II–III are suitable for surgery with favorable outcomes. Although patients with cardiac function class IV face higher surgical risks, they should undergo surgery after bed rest and medical treatment to control heart failure and improve their condition. Cases with pulmonary {|###|}stirred pulse hypertension may still be considered for surgery. Systemic peripheral embolism should be treated surgically after removing the thrombus in the {|###|}stirred pulse. Cerebral embolism requires waiting several weeks until the condition stabilizes before surgery. Recurrent {|###|}wind-dampness heat and bacterial endocarditis should delay surgical treatment. Acute pulmonary {|###|}edema and massive hemoptysis uncontrolled by medical therapy may require emergency surgery. Pregnant women with mitral stenosis should undergo surgery early in {|###|}pregnancy to avoid increased cardiac burden during the late stage [third stage] when circulating blood volume rises. Patients with grade I functional tricuspid regurgitation should undergo mitral valve dilation and separation, as tricuspid regurgitation often improves or resolves postoperatively. Severe functional tricuspid regurgitation or organic {|###|}sexually transmitted disease changes in the tricuspid valve require tricuspid valve repair alongside mitral stenosis relief.
Surgical history: Cutler and Levine first inserted a specialized curved knife via the left ventricular apex to incise mitral stenosis in 1902. In 1925, Souttar used a finger to separate and enlarge the mitral valve orifice via the left atrial appendage. From 1947 to 1948, Harken, Bailey, and Brock performed closed mitral commissurotomy via the left atrial appendage with good outcomes. In 1954, Neptune and Bailey reported mitral commissurotomy via a right atrial incision, avoiding intraoperative dislodgement of left atrial appendage thrombi and reducing embolism risk in atrial fibrillation patients. From 1954 to 1960, Beck, Glenn, Logan, Turner, and Tubbs developed mechanical mitral dilators inserted via small left atrial or ventricular incisions to enlarge the valve orifice under finger guidance, improving outcomes. Starting in 1957, Lillehei, Merendino, and Bailey performed open-heart mitral valve surgery under cardiopulmonary bypass, enabling precise separation of fused valve {|###|}membrane commissures and chordae, improving leaflet mobility, and removing calcified lesions and atrial thrombi. This method yielded better results than closed commissurotomy and reduced embolism risk but required advanced equipment, more manpower, and higher costs. In 1961, Starr and Edwards successfully performed the first artificial mitral valve replacement, enabling surgery for patients with grade III mitral stenosis and severe valve {|###|}membrane sclerosis or calcification.
Selection of surgical methods: Closed mitral valve commissurotomy: The mitral valve dilator is used to separate the adherent valve membrane and enlarge the valve orifice. The procedure is relatively simple and has good therapeutic outcomes. Currently, this surgical method is still widely used for most cases of mitral stenosis in China. Closed surgery is suitable for cases of pure mitral stenosis where the valve membrane lesion is of the septal membrane type, with no significant leaflet thickening and good mobility. For cases suspected of having left atrial thrombosis, a right anterior thoracotomy is preferred, inserting fingers and the dilator into the left atrium through the interatrial groove. Closed mitral valve commissurotomy is also suitable for cases with concomitant grade I functional tricuspid regurgitation where the tricuspid valve lesion does not require intervention.
Direct vision mitral commissurotomy: Suitable for all types of mitral stenosis cases. Since it allows precise incision of the fused valve commissures, separation of subvalvular chordae tendineae and papillary muscle adhesions, and removal of calcifications and left atrial thrombi, the therapeutic outcomes are most satisfactory. However, due to current limitations in medical conditions in our country, direct vision surgery is mostly applied to cases with left atrial thrombi, mitral restenosis, those highly suspected of concomitant mitral insufficiency potentially requiring valve replacement, as well as cases complicated by grade III functional tricuspid insufficiency or organic tricuspid lesions requiring simultaneous correction.
Prosthetic valve replacement: Suitable for cases with severely damaged valves and those accompanied by moderate or greater mitral insufficiency. Cases complicated by grade III tricuspid insufficiency require simultaneous tricuspid valvuloplasty or replacement.
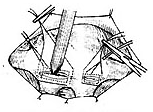
Operative Technique: Closed Mitral Commissurotomy: Four types of thoracic incisions can be used: left anterolateral thoracotomy; left posterolateral thoracotomy; right anterior thoracotomy; and median sternotomy. For the left anterior thoracotomy approach, the patient is placed in a supine position with the left back elevated by 30°, the left arm extended forward and upward, and the elbow flexed at 90° and fixed suspended above the head. An incision below the breast is made through the 4th or 5th intercostal space to enter the pleural cavity. The left lung is retracted laterally to expose the pericardium. The pericardium is incised longitudinally about 1–2 cm anterior or posterior to the phrenic nerve, parallel to the nerve, extending superiorly to the upper edge of the lung and inferiorly to the diaphragm. The edges of the incision are coagulated for hemostasis. The posterior edge of the pericardium is sutured to a gauze pad and retracted laterally. The anterior edge of the pericardium is sutured to the anterior chest wall with several stitches to expose the left atrial appendage and the apex of the left ventricle. In cases of mitral stenosis, a diastolic thrill can be palpated at the apex, while a systolic thrill palpated in the left atrium suggests mitral regurgitation. A systolic thrill at the aortic root indicates aortic valve stenosis. A purse-string suture is placed at the base of the left atrial appendage, with both ends fixed to a Rumel tourniquet. Another pledgeted mattress suture is placed in an avascular area at the apex of the left ventricle, with both ends clamped by mosquito forceps. The suture should pass through most of the myocardium but not enter the ventricular cavity, with the sutures spaced 0.8 cm apart to accommodate the mitral dilator. The atrial clamp is used to grasp the left atrial wall at the base of the appendage, and the appendage wall is incised, cutting the trabeculae inside the appendage. The surgeon’s right glove is removed, and the index finger is sterilized sequentially with 3% iodine tincture, 75% alcohol, and 3.8% sodium citrate solution. An assistant gently retracts the edges of the appendage incision with Allis forceps to open it. The surgeon releases the atrial clamp with the left hand while inserting the right index finger into the left atrium through the appendage incision. The assistant immediately tightens the purse-string suture to prevent bleeding. The finger explores the mitral valve for mobility, degree of thickening, calcification, valve orifice size, and presence of regurgitation. A small incision is made with a scalpel in the mattress suture area at the apex, penetrating the full thickness of the myocardium. The mitral dilator is advanced along the left ventricular inflow tract, guided by the finger in the atrium, with about 1/3 to 1/2 of the dilator tip passing through the mitral valve into the left atrium. The surgeon controls the dilator handle with the left hand, forcefully opening the dilator to separate the fused commissures (Figure 1). The valve orifice should be enlarged in 2–3 stages, starting from 2.0–2.5 cm and gradually expanding to 3.0 or 3.5 cm. After each dilation, the handle is immediately released to close the dilator, and the tip is withdrawn into the left ventricle. The finger checks the enlarged orifice and any resulting regurgitation. If no regurgitation occurs, the dilator handle’s screw ring is adjusted to modify the dilation amplitude, and the dilator is reintroduced into the mitral orifice for further dilation. If mitral regurgitation occurs after dilation, further enlargement should be avoided. After completing the dilation, the dilator is removed, and the apical mattress suture is tied, with additional interrupted sutures if needed. The finger is withdrawn from the left atrium while the atrial clamp grasps the base of the appendage, and the appendage incision is sutured or the base ligated. The pericardial cavity is irrigated with saline, and a small pericardial incision is made posterior to the phrenic nerve near the diaphragm for drainage. The pericardium is loosely sutured, a chest tube is placed, and the thoracic incision is closed in layers. For the left posterolateral thoracotomy approach, the patient is placed in a lateral decubitus position with the upper body slightly reclined. The incision is made through the 5th intercostal space, and the intracardiac procedures are the same as for the left anterior thoracotomy. The posterolateral approach provides better exposure, and sometimes 2–3 paraffin gauze pads are placed behind the apex to elevate the left ventricular apex for easier dilator insertion.
(1) Insert the dilator under finger guidance; (2) Dilate and separate the commissure of the valve membrane
Figure 1: Closed Mitral Commissurotomy
Right Thoracic Approach: The patient is placed in a supine position with the right back elevated by 30°, tilting the body to the left side. The right arm and elbow are suspended and fixed above the head. An incision is made below the breast from the parasternal to the midaxillary line on the right anterior chest, entering the thorax through the fourth intercostal space. The pericardium is incised longitudinally about 2 cm anterior to the right phrenic nerve, with the upper end of the incision reaching the level of the great vessels at the base of the heart and the lower end reaching the diaphragm. The anterior edge of the pericardium is sutured and fixed to the anterior chest wall, while the posterior edge is sutured to a gauze pad for traction. The interatrial groove is dissected at two sites to separate the interface between the left and right atria. The upper dissection area is 1.5–2 cm long, and the lower dissection area is about 1.0 cm long, with approximately 1 cm of the interatrial groove left undissected between the two areas. Two layers of purse-string sutures are placed in each dissected area of the interatrial groove. The inner layer of purse-string sutures is fixed at both ends to a Rumel tourniquet, while the outer layer is clamped with mosquito forceps. A small incision is first made in the left atrial wall within the upper purse-string suture area using a small round knife. The surgeon’s left index finger is inserted through the incision into the left atrium for exploration. If the mitral valve pathology is deemed suitable for commissurotomy, a right-sided dilator with a more curved tip is inserted through the lower dissection area. Under the guidance of the surgeon’s finger, the dilator enters the mitral valve orifice, and the fused commissures are separated incrementally to enlarge the valve orifice (Figure 2). After dilation, the dilator is removed, and the purse-string sutures are tied. If necessary, 1–2 additional sutures are added. The finger is then withdrawn, and the purse-string sutures are tied. The pericardial incision is loosely sutured, with the lower end left unsutured for pericardial drainage. A chest tube is placed, and the chest wall incision is closed layer by layer. Performing mitral commissurotomy via the interatrial groove left atrial incision avoids manipulation of the left atrial appendage, reducing the incidence of thromboembolic complications. This approach is suitable for cases with chronic atrial fibrillation, chest X-rays showing a concave atrial appendage suggesting a small left atrial appendage, or cases of restenosis or left-sided membrane thickening after previous left thoracotomy for commissurotomy. However, in cases with a significantly enlarged left atrium, the right thoracic approach may make it difficult for the finger to adequately explore the mitral valve orifice, and controlling the direction and position of the dilator may also be challenging.
(1) Place two purse-string and "U" sutures
(2) Puncture the left atrium and create an opening with a metal cone
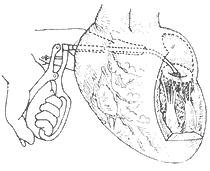
(3) Dilate the mitral valve via the interatrial groove
Figure 2: Right Thoracic Interatrial Groove Approach for Mitral Commissurotomy
Median sternotomy approach: The patient is placed in a supine position with the upper back slightly elevated. A midline incision is made along the anterior chest, and the sternum is longitudinally divided to enter the thoracic cavity. The pleura is retracted bilaterally, and an "I"-shaped incision is made in the anterior midportion of the pericardium. Two to three pieces of paraffin gauze are placed posterior and to the left of the heart to elevate the apex. The interatrial groove is dissected to expose the anterior wall of the left atrium, where two concentric purse-string sutures are placed. The inner suture ends are secured to a Rumel tourniquet, while the outer purse-string suture ends are clamped with mosquito forceps. A single pledgeted mattress suture is placed in the avascular area of the left ventricular apex, with both ends clamped by mosquito forceps. The surgeon’s left index finger is inserted through the left atrial incision for intracardiac exploration. If the mitral valve pathology is suitable for commissurotomy, a dilator is introduced through the left ventricular apex to gradually expand the mitral valve orifice to 3.0–3.5 cm. The dilator is then removed, and the left ventricular incision is closed. After withdrawing the index finger, the interatrial groove purse-string sutures are tied. The pericardial incision is loosely closed, leaving a drainage opening at the lower end. Pericardial and thoracic drainage tubes are placed, and the sternum is reapproximated and fixed with stainless steel wires. The incision is closed in layers. The median sternotomy approach is indicated for cases of chronic atrial fibrillation, suspected left atrial thrombus, recurrent mitral stenosis after previous dilation, or when direct vision mitral commissurotomy may be required.
Open Mitral Commissurotomy: Open mitral commissurotomy can precisely incise the fused valve leaflets, effectively relieve subvalvular stenosis, satisfactorily improve valve mobility, remove left atrial thrombi and calcified valve lesions, and restore valve function. The postoperative restenosis rate is very low. For cases with moderate or greater functional tricuspid regurgitation, tricuspid annuloplasty can be performed simultaneously, and for those with organic tricuspid valve disease, tricuspid valve replacement can also be done concurrently. Due to improvements in extracorporeal circulation techniques and equipment, the surgical mortality rate of open mitral commissurotomy has become comparable to that of closed mitral commissurotomy. Therefore, when equipment, technical capabilities, and medical costs permit, open mitral commissurotomy should be considered the preferred surgical treatment for mitral stenosis.
The patient is placed in the supine position. A midline incision is made on the anterior chest, and the sternum is longitudinally sawed open. The pleura is pushed aside, the pericardium is incised, and the heart is exposed. A finger is inserted through the right atrial appendage incision to explore whether the tricuspid valve is accompanied by insufficiency. Then, the superior and inferior vena cava drainage catheters are inserted through the right atrial appendage and right atrial incisions, respectively, or a single thicker drainage catheter is placed in the right atrium. A perfusion catheter is inserted into the ascending aorta. After establishing extracorporeal circulation, the body temperature is lowered to approximately 25°C, while cold saline is injected into the pericardial cavity to further reduce the local temperature of the heart. The ascending aorta is clamped, and cold cardioplegic solution is injected under pressure at its root. The interatrial groove is dissected, and a longitudinal incision is made on the anterior wall of the left atrium to insert a left atrial retractor. If there are thrombi in the left atrium, they must be completely removed. Before removal, a small gauze is placed at the mitral valve orifice to prevent thrombi from entering the left ventricle. After removing the thrombi, the blood in the left atrium is suctioned out with an aspirator, and the left atrium is rinsed with saline to thoroughly remove any residual thrombus fragments. The mitral valve leaflets and subvalvular lesions are inspected. If the leaflets are severely thickened, calcified, rigid, have poor mobility, or are accompanied by moderate or greater insufficiency, mitral valve replacement should be considered. If the valve is not calcified or only mildly calcified and not accompanied by insufficiency, mitral valve stenosis separation can be performed. Traction sutures are placed at the edges of the anterior and posterior leaflets, respectively, and a right-angle vascular clamp is used to grasp the sutures and lift the leaflets upward to open the valve orifice. The fused commissures will appear as thickened wrinkles, making them easy to identify. Another right-angle vascular clamp is placed between the chordae tendineae below the commissure, gently lifting and spreading the fused commissure to clearly expose it and avoid injuring the chordae tendineae. A small round blade is used to precisely incise the fused commissure. After each 2–3 mm incision, the right-angle vascular clamp is reinserted. Both commissures can be incised to within 1–2 mm of the annulus without causing insufficiency. After incising the fused commissures, the mobility of the valve is carefully inspected. If chordal adhesions mildly affect valve mobility, a nerve hook can be used to separate them. For tightly fused and shortened chordae, the chordae and part of the papillary muscle tips are incised and separated. In cases where leaflet calcification affects mobility, the calcifications are carefully scraped off, taking care to avoid injuring the leaflet tissue and preventing calcific debris from scattering in the left heart chamber. After completing the mitral stenosis open separation, the mitral valve's closing function is tested. One testing method involves inserting an F10 plastic tube through the ascending aortic wall. The tube has multiple small side holes distributed over a 6–7 cm length. The catheter is inserted through the aortic valve orifice into the left ventricular cavity, with some side holes in the ascending aorta and others in the left ventricle. The external end of the catheter is clamped, and the aortic clamp is released, allowing blood to fill the left ventricle through the side holes. Observe whether blood regurgitates from the mitral valve into the left atrium (Figure 3). Alternatively, a catheter can be inserted into the left ventricle through the apex, and saline is injected to fill the left ventricle to observe for mitral regurgitation. Due to the myocardium being in a relaxed state without contractile function, these two testing methods are less reliable. Stopping extracorporeal circulation and allowing the heart to resume beating, with systolic blood pressure reaching above 12 kPa (90 mmHg), finger exploration of the mitral valve through the left atrium for regurgitation is more reliable. When extracorporeal circulation is stopped, systolic blood pressure is above 12 kPa (90 mmHg), and the cardiac index reaches 3 L/(min·m²), measuring the left atrial-left ventricular diastolic pressure gradient can determine whether the mitral stenosis separation was successful.
Figure 3 Open Mitral Commissurotomy
(1) Dissect the interatrial groove and incise the left atrium; (2) Expose the mitral valve; (3) Incise the anterior commissure; (4) Incise the posterior commissure; (5) Separate the chordal adhesions; (6) Suture the left atrial incision
After completing the open mitral commissurotomy, use a 4-0 non-traumatic suture to perform a double-layer continuous suture at the base of the left atrial appendage to close it and prevent thrombus formation. Care should be taken to avoid deep needle penetration that could injure the circumflex branch of the coronary artery in the atrioventricular groove. After evacuating residual air from the left atrium, suture the left atrial incision. Insert an air vent needle into the ascending aorta, rewarm until the body temperature reaches above 35°C, and stop extracorporeal circulation once the heart beats strongly. Remove the right atrial or superior/inferior vena cava drainage cannula and the ascending aorta perfusion cannula. Achieve hemostasis and suture the pericardium, sternum, and chest wall incisions.
Mitral Valve Replacement: For cases where the mitral valve tissue is severely calcified, leaflet mobility is lost, or mitral stenosis is accompanied by grade III insufficiency, making commissurotomy unlikely to improve valve function, the diseased mitral valve must be excised and replaced with a mechanical or bioprosthetic valve. Artificial valves have been in clinical use for 30 years, and although continuous improvements have been made, they are still not perfect. Mechanical valves have a high incidence of thromboembolic complications and require long-term anticoagulation postoperatively. The durability of bioprosthetic valves remains unsatisfactory. Currently, the surgical mortality rate for valve replacement is also higher than that of closed or open mitral commissurotomy. Therefore, the indications for mitral valve replacement must be strictly adhered to. Details regarding the procedure of mitral valve replacement, valve selection, and potential postoperative complications.
Therapeutic effect: The surgical mortality rate of closed mitral commissurotomy is approximately 2%. The long-term postoperative efficacy is related to factors such as the severity of the valvular lesion, the cardiac function grade, the degree of valve orifice enlargement, the presence of pre-existing or surgically induced mitral regurgitation, and postoperative rheumatic activity leading to mitral restenosis. In the early postoperative period, clinical symptoms disappear or significantly improve in about 80% of cases, with cardiac function improving to grade I–II. However, after 10–15 years, symptoms recur or gradually worsen in approximately 30–50% of cases. The incidence of restenosis after commissurotomy is 10–30%, primarily due to inadequate separation of valvular commissural adhesions and frequent postoperative rheumatic activity. The postoperative thromboembolic complication rate is nearly 10%. The 10-year postoperative mortality rate is 30–40%.
The operative mortality rate for direct vision mitral valve commissurotomy is less than 2%, similar to that of closed mitral valvotomy. Since both valvular and subvalvular lesions can be properly corrected, 90% of patients postoperatively achieve cardiac function improvement to grade I-II. The thromboembolic complication rate is low, approximately 0.3% per year. Only 6% of cases require reoperation due to restenosis. At 5 years postoperatively, 80% of patients are free from complications, and at 10 years, about 66% remain complication-free. The mortality rate for advanced stage cases is 2.5%. At 10 years postoperatively, 80-90% of patients survive.
The operative mortality rate for mitral valve replacement in cases of rheumatic mitral stenosis is approximately 7-10%. The 10-year survival rate postoperatively is 60-70%. For those receiving mechanical valves, only 20-30% remain free from complications at 10 years. In cases using bioprosthetic valves, 70% of patients are complication-free at 5-year follow-up, but the durability of bioprosthetic valves remains a concern.
Diagnosing mitral stenosis is generally not difficult. Typical pure mitral stenosis can be clearly diagnosed based on medical history and signs. The clinical manifestations and cardiac signs of left atrial myxoma are extremely similar to those of rheumatic mitral stenosis. The heart murmur in cases of left atrial myxoma may change in intensity or disappear with changes in body position. Echocardiography can show the cloud-like echo reflection of the tumor in the left atrium entering the mitral valve orifice or left ventricle during diastole and retracting back into the left atrium during systole, which is highly valuable for confirming the diagnosis. For mitral stenosis cases considered for surgical treatment, it is also necessary to determine whether mitral regurgitation is present and whether other valve membranes are affected, as well as the severity of the lesions. For patients over 40 years old, selective coronary angiography is recommended to assess whether there are obstructive lesions in the coronary arteries.





