| disease | Hypertrophic Cardiomyopathy |
The characteristic feature of this type is ventricular myocardial hypertrophy, typically in the left ventricle, especially in the interventricular septum, and occasionally presenting as concentric hypertrophy. The volume of the left ventricular cavity is normal or reduced. Occasionally, the disease occurs in the right ventricle. It is usually inherited in an autosomal dominant manner.
bubble_chart Etiology
The cause of the disease is unknown. Currently, genetic factors are considered the primary cause, supported by the evident familial predisposition of the disease, frequent association with other congenital cardiovascular anomalies, and the presence of the disease at birth in some patients. Genetic HLA antigen genotypes have been observed in affected individuals. Familial cases are transmitted in an autosomal dominant pattern, manifesting either as asymptomatic asymmetric myocardial hypertrophy or with typical obstructive symptoms. Several hypotheses exist regarding the mechanisms by which genetic defects lead to the disease: ① Abnormal catecholamines and sympathetic nervous system activity, evidenced by frequent associations with neural crest disorders, hyperthyroidism, excessive insulin secretion, hypertension, and responsiveness to beta-blockers. ② Disproportionate thickening of the fetal ventricular septum and disordered myocardial fiber arrangement that fails to regress normally after birth. ③ Accelerated atrioventricular conduction causing asynchronous excitation and contraction between the ventricular septum and left ventricular free wall. ④ Primary collagen abnormalities leading to disordered cardiac fibrous scaffolding and misalignment of myocardial fibers. ⑤ Abnormal myocardial protein synthesis. ⑥ Abnormal small coronary artery pulsation, resulting in ischemia, fibrosis, and compensatory myocardial hypertrophy. ⑦ The ventricular septum bulges leftward in the transverse plane and leftward along the apex-base axis (normally concave leftward), causing unequal contraction lengths, disordered myocardial fiber arrangement, and localized hypertrophy. The pathogenic mechanisms of sporadic cases without familial or genetic evidence remain unclear.
bubble_chart Pathological ChangesThe pathological changes are primarily characterized by myocardial hypertrophy, with an increase in heart weight. Myocardial hypertrophy can be observed in the interventricular septum and free wall, with the former being more pronounced and often exhibiting asymmetric (non-concentric) hypertrophy, meaning the degree of hypertrophy varies across different regions of the ventricular wall. The left ventricle is commonly affected, while the right ventricle is rarely involved. When the interventricular septum is severely hypertrophied and protrudes into the left ventricular cavity, causing obstruction of the left ventricular outflow tract during systole, it is termed "hypertrophic obstructive cardiomyopathy," formerly known as "idiopathic hypertrophic subaortic stenosis." If the hypertrophy of the interventricular septum is milder and does not cause significant obstruction of the left ventricular outflow tract during systole, it is referred to as "hypertrophic non-obstructive cardiomyopathy." The anterior papillary muscle may also become hypertrophied, often displacing and impairing normal valve function. In cases of severe myocardial hypertrophy, the left ventricular cavity may shrink. Disproportionate myocardial hypertrophy often results in a ratio of interventricular septum thickness to left ventricular posterior wall thickness of ≥1.3, with some cases reaching up to 3. A variant of hypertrophic cardiomyopathy involves prominent myocardial hypertrophy in the apical region. In this variant, the subepicardial coronary arteries are normal, but the intramural coronary arteries are increased in number with narrowed lumens. Microscopically, the myocardial cells exhibit disordered arrangement, abnormal nuclei, increased branching, and heightened mitochondrial content. The myocardial cells are extremely hypertrophied, with increased intracellular glycogen content, along with interstitial fibrosis. Electron microscopy reveals disordered myofibril arrangement. In two-thirds of patients, the mitral valve leaflets are enlarged and elongated, and a fibrous patch is present on the left ventricular endocardial wall opposite the anterior mitral leaflet, caused by the impact of the mitral valve against the interventricular septum. This condition can occur at any age, but myocardial hypertrophy tends to be more severe in individuals under 40 years old compared to those over 40, though the reason for this age-related difference remains unclear. As the disease progresses, myocardial fibrosis increases, ventricular wall hypertrophy decreases, and the degree of ventricular cavity narrowing lessens, presenting as an advanced-stage manifestation.
1. Left ventricular outflow tract obstruction—During systole, hypertrophied myocardium narrows the ventricular outflow tract. In non-obstructive cases, this effect is less pronounced, whereas it is more prominent in obstructive cases. During ventricular contraction, the hypertrophied interventricular septum bulges into the ventricular cavity, causing the anterior mitral leaflet in the outflow tract to move closer to the septum and shift forward, leading to left ventricular outflow tract obstruction and mitral regurgitation. This effect is more noticeable during mid-to-late systole. In early left ventricular ejection, outflow tract obstruction is mild, allowing approximately 30% of the stroke volume to be ejected, while the remaining 70% is ejected when obstruction becomes significant. As a result, the carotid pulse wave shows a rapidly rising upstroke, followed by a downward deflection and a secondary rise forming a notch, then a slow descent. Outflow tract obstruction during systole creates a pressure gradient between the left ventricular cavity and the outflow tract, but no pressure gradient exists between the outflow tract and the aorta. In some patients, outflow tract obstruction is not apparent at rest but becomes evident during exercise.
2. Diastolic dysfunction—Hypertrophied myocardium exhibits reduced compliance and impaired expansion, leading to impaired ventricular diastolic filling and potentially elevated end-diastolic pressure. The increased stiffness of the diastolic chamber and reduced left ventricular expansion result in decreased stroke volume, elevated filling pressure, and compression of the intramural coronary arteries. The rapid filling phase is prolonged, with reduced filling rate and volume.
bubble_chart Clinical Manifestations
The onset is usually gradual. About one-third of cases have a family history. Symptoms mostly begin before the age of 30. Both sexes are equally affected.
The main symptoms are: ① Dyspnea, often occurring after exertion, is due to reduced left ventricular compliance, elevated end-diastolic pressure, and subsequent pulmonary venous pressure increase, leading to pulmonary static blood. Mitral regurgitation associated with ventricular septal hypertrophy can exacerbate pulmonary static blood. ② Precordial pain, often occurring after exertion, resembles cardiac colicky pain but may be atypical, caused by increased oxygen demand in hypertrophied myocardium and relative insufficiency of coronary stirred pulse blood supply. ③ Lack of strength, dizziness, and syncope, often occurring during activity, result from increased heart rate further shortening the already inadequate left ventricular diastolic filling period, worsening filling insufficiency and reducing cardiac output. During activity or emotional stress, sympathetic stimulation strengthens the contraction of hypertrophied myocardium, worsening outflow tract obstruction and causing a sudden drop in cardiac output, leading to symptoms. ④ Palpitation, due to impaired cardiac function or arrhythmia. ⑤ Heart failure, commonly seen in advanced stage patients, results from reduced myocardial compliance, significantly elevated ventricular end-diastolic pressure, subsequent atrial pressure increase, and frequent atrial fibrillation. Advanced stage patients have extensive myocardial fibrosis, weakened ventricular systolic function, and are prone to heart failure and sudden death.
Common signs include: ① The cardiac dullness border expands to the left. The apical impulse shifts leftward and downward, with a lifting impulse. Alternatively, a double apical impulse may be felt, caused by atrial contraction into the less compliant ventricle before the apical impulse. ② A systolic intermediate stage [second stage] or advanced stage ejection murmur, heard at the lower left sternal border near the apex, radiating to the apex but not the base, may accompany a systolic tremor in patients with ventricular outflow tract obstruction. Measures that increase myocardial contractility or reduce cardiac load—such as administering Rehmannia glycosides, isoproterenol (2µg/min), amyl nitrite, nitroglycerin, performing the Valsalva maneuver, after physical exertion, or post-premature beats—can intensify the murmur. Conversely, measures that weaken myocardial contractility or increase cardiac load—such as vasoconstrictors, β-blockers, squatting, or handgrip—can diminish the murmur. About half of patients also exhibit a mitral regurgitation murmur. ③ The second heart sound may show paradoxical splitting due to delayed closure of the aortic stirred pulse valve caused by left ventricular ejection obstruction. A third heart sound is common in patients with mitral regurgitation.
bubble_chart Auxiliary Examination
Electrocardiographic manifestations: ① ST-T changes are observed in over 80% of patients. Most have normal coronary pulses, while a few patients with localized myocardial hypertrophy in the apical region exhibit giant inverted T waves due to abnormal coronary pulses. ② Signs of left ventricular hypertrophy are seen in 60% of patients, and their presence is related to the degree and location of myocardial hypertrophy. ③ The presence of abnormal Q waves. Deep but not wide Q waves in leads V6, V5, aVL, and I reflect asymmetric septal hypertrophy and should not be mistaken for myocardial infarction. Q waves may also appear in leads II, III, aVF, V1, and V2, likely due to irregular impulses and delayed conduction in the subendocardial and intramural myocardium following left ventricular hypertrophy. ④ Abnormal left atrial waveforms may be observed in one-fourth of patients. ⑤ Some patients may also have pre-excitation syndrome (Figure 1).
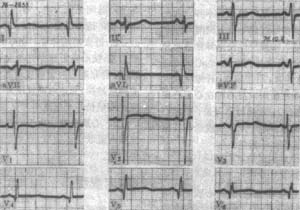
Figure 1 Electrocardiogram of hypertrophic primary cardiomyopathy
Abnormal Q waves in leads I, aVL, V4, V5, and V6 are caused by the larger initial rightward depolarization vector due to hypertrophied ventricular septum. Leads V1 and V2 show RS patterns with higher R waves, corresponding to the Q waves in the aforementioned leads. Echocardiography in this case revealed ventricular septal hypertrophy.
Echocardiographic manifestations: ① Asymmetric septal hypertrophy, with a septal-to-posterior wall thickness ratio greater than 1.3:1. While this sign was previously emphasized, it is now recognized that it can also occur in other conditions such as hypertension and aortic stenosis. Two-dimensional measurement of left ventricular thickening is more useful. ② Anterior mitral leaflet systolic anterior motion. ③ Reduced left ventricular cavity size and narrowed outflow tract. ④ Left ventricular diastolic dysfunction, including decreased compliance, prolonged rapid filling time, and prolonged isovolumic relaxation time. Doppler methods can identify the origin of murmurs and calculate pressure gradients before and after obstruction (Figure 2).
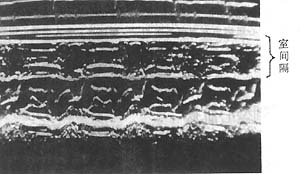
Figure 2 M-mode echocardiogram of hypertrophic primary cardiomyopathy
The image shows significant septal thickening, upward flipping of the CD segment, and marked narrowing of the left ventricular outflow tract. The diagnosis in this case was confirmed surgically.
Plain chest X-rays may show left ventricular enlargement or appear normal. X-ray or radionuclide angiography can demonstrate septal thickening and reduced left ventricular cavity size. Radionuclide myocardial scanning can reveal the location and extent of myocardial hypertrophy.
Cardiac catheterization reveals elevated ventricular end-diastolic pressure. In cases with left ventricular outflow tract obstruction, a systolic pressure gradient exists between the ventricular cavity and the outflow tract.
Patients with ventricular outflow tract obstruction are not difficult to diagnose due to their characteristic clinical manifestations. Echocardiography is an extremely important non-invasive diagnostic method, helpful for both obstructive and non-obstructive patients. A ventricular septal thickness ≥18mm combined with systolic anterior motion of the mitral valve is sufficient to distinguish obstructive from non-obstructive sexually transmitted disease cases. Cardiac catheterization revealing a left ventricular outflow tract pressure gradient can confirm the diagnosis. Ventriculography also has diagnostic value. Clinically, the presence of a systolic murmur at the lower left sternal border should raise suspicion for this condition. Observing changes in the murmur through physiological maneuvers or pharmacological interventions affecting hemodynamics aids in diagnosis. Additionally, the following differential diagnoses must be considered.
(1) Ventricular septal defect: The systolic murmur location is similar, but it is pansystolic, and the apical region usually lacks a murmur. Echocardiography, cardiac catheterization, and heart blood vessel angiography can differentiate.
(2) Main stirred pulse valve stenosis: Symptoms and murmur characteristics are similar, but the murmur is located higher, often accompanied by a systolic ejection sound at the main stirred pulse valve area, weakened second heart sound, and possibly an early diastolic murmur. X-ray shows dilation of the ascending main stirred pulse. Physiological maneuvers or pharmacological interventions have little effect on the murmur. Left heart catheterization reveals a systolic pressure gradient across the main stirred pulse valve. Echocardiography can pinpoint the lesion site.
(3) Wind-dampness-related mitral regurgitation: The murmur is similar but usually pansystolic, intensified by vasoconstrictors or squatting, often accompanied by atrial fibrillation and a larger left atrium. Echocardiography does not show a ventricular septal defect.
(4) Coronary heart disease: Both share symptoms like colicky pain and ECG findings such as ST-T changes and abnormal Q waves. However, coronary heart disease lacks characteristic murmurs, the main stirred pulse is often widened or calcified, and hypertension and hyperlipidemia are common. Echocardiography shows no ventricular septal thickening but may reveal segmental wall motion abnormalities.
bubble_chart Treatment Measures
Since the cause of the disease is unclear, prevention is difficult. After echocardiography detects cases of latent sexually transmitted diseases, genetic data can be used for research. To prevent the onset of the disease, it is advisable to avoid fatigue, agitation, and sudden exertion. Drugs that enhance myocardial contractility, such as digitalis, β-receptor stimulants like isoproterenol, and those that reduce cardiac load, such as nitroglycerin, should be avoided as they worsen left ventricular outflow tract obstruction. If mitral valve insufficiency is present, preventive measures against infective endocarditis should be taken.
The treatment goals are to alleviate symptoms and control arrhythmias. Current treatments include: ① β-receptor blockers, which weaken myocardial contraction, reduce outflow tract obstruction, decrease myocardial oxygen consumption, enhance diastolic ventricular expansion, and slow the heart rate while increasing stroke volume. Propranolol was the earliest used, starting at 10mg per dose, 3–4 times daily, with gradual dose increases to improve symptoms without excessively lowering heart rate or blood pressure, up to around 200mg/day. However, recent findings suggest that β-receptor blockers do not reduce arrhythmias or sudden death, nor do they improve prognosis. ② Calcium antagonists have both negative inotropic effects to weaken myocardial contraction and improve myocardial compliance, benefiting diastolic function. Verapamil, at 120–480mg/day divided into 3–4 oral doses, can provide long-term symptom relief but should be used cautiously in patients with hypotension, sinus node dysfunction, or atrioventricular conduction disorders. Diltiazem is also effective, with a dosage of 30–60mg, three times daily. Combining β-receptor blockers with calcium antagonists may reduce side effects and enhance efficacy. ③ Antiarrhythmic drugs, such as amiodarone, are commonly used to control rapid ventricular arrhythmias and atrial fibrillation. Electrical cardioversion may be considered if drug therapy fails. ④ For advanced-stage patients with impaired ventricular systolic function leading to congestive heart failure, treatment is similar to that for heart failure caused by other conditions. For patients with a confirmed diagnosis and poor response to drug therapy, surgical options such as septal myectomy or partial resection of hypertrophic myocardium may be considered to relieve symptoms. Recently, dual-chamber permanent pacemakers have been trialed for right ventricular atrioventricular sequential pacing to alleviate symptoms in obstructive cases, though further experience is needed.
The disease progresses slowly with an uncertain prognosis. It can remain stable for many years without change, but once symptoms appear, it can gradually worsen. Sudden death and heart failure are the primary causes of death. Sudden death is more common in children and young adults, and its occurrence is related to physical activity, the presence or absence of symptoms or obstruction, the degree of ventricular wall thickening, a family history of sudden death, and persistent ventricular tachycardia, which are risk factors for sudden death. The possible mechanisms of sudden death include rapid ventricular arrhythmias, sinus node dysfunction and conduction disorders, myocardial ischemia, diastolic dysfunction, and hypotension, with the first two being the most critical. The onset of atrial fibrillation can exacerbate heart failure. A small number of patients may experience complications such as infective endocarditis or embolism.





