| disease | Neonatal Hyaline Membrane Disease |
| alias | Neonatal Respiratory Distress Syndrome, Neonatal Respiratory Distress Syndrome, NRDS, Hyaline Membrane Disease, HMD |
Neonatal pulmonary hyaline membrane disease (hyaline membrane disease, HMD), also known as neonatal respiratory distress syndrome (NRDS), refers to the onset of progressive dyspnea, cyanosis, expiratory moaning, inspiratory retractions, and respiratory failure shortly after birth. It is primarily seen in premature infants due to insufficient pulmonary surfactant leading to progressive atelectasis. The pathological feature is the presence of eosinophilic hyaline membranes lining the alveolar walls and terminal bronchioles.
bubble_chart Etiology
This disease is caused by a deficiency of pulmonary surfactant (PS) produced by type II alveolar cells. Over 80% of pulmonary surfactant is composed of phospholipids (PL), which appear between 20 to 24 weeks of gestation and increase rapidly after 35 weeks. Therefore, this condition is more common in premature infants, with the incidence increasing the earlier the gestational age. The causes of pulmonary surfactant (PS) deficiency include: ① Prematurity: In infants born before 35 weeks, type II cells are underdeveloped, leading to insufficient PS production; ② Hypoxia, acidosis, and hypothermia: These conditions can inhibit the synthesis of PS in premature infants after birth; ③ Infants of diabetic mothers: These infants experience pancreatic islet cell hyperplasia, and insulin antagonizes the effects of adrenal corticosteroids, delaying lung maturation; ④ Cesarean section: The lack of normal uterine contractions reduces the stimulation of adrenal corticosteroids, which promote lung maturation, resulting in relatively less PS; ⑤ Ventilation abnormalities: These can interfere with PS synthesis; ⑥ Pulmonary infections: Type II cells are damaged, reducing PS production.
bubble_chart PathogenesisSurfactant can reduce the surface tension at the interface between the alveolar wall and the gas within the alveoli, keeping the alveoli open. Its short half-life necessitates continuous replenishment. When surfactant is deficient, the surface tension of the alveoli increases. According to the formula P (alveolar recoil pressure) = 2T (surface tension) / r (alveolar radius), the smallest alveoli collapse first during exhalation, leading to progressive atelectasis. This results in worsening clinical symptoms such as respiratory distress and cyanosis. The process is as follows: insufficient alveolar surfactant → increased surface tension of the alveolar wall (increased alveolar recoil) → collapse of the smallest alveoli first → progressive atelectasis → hypoxia and acidosis → pulmonary arteriole spasm → increased pulmonary artery pressure → opening of the foramen ovale and ductus arteriosus → right-to-left shunting (persistent fetal circulation) → decreased pulmonary blood flow → worsening hypoxia in lung tissue → increased capillary permeability → fibrin deposition → formation of hyaline membranes → further exacerbation of hypoxia and acidosis, creating a vicious cycle.
bubble_chart Pathological Changes
Gross: Both lungs appear dark red with sharp margins and a liver-like consistency. The lateral edges often show rib impressions. Large or small pieces of lung tissue sink in water, and pale red bloody fluid can often be seen exuding from bronchial openings when the lungs are squeezed.
Microscopic: Pulmonary arterioles are constricted, with capillaries and venules showing static blood. The majority of alveoli are collapsed, exhibiting extensive resorptive atelectasis. The characteristic lesion consists of a transparent, homogeneous, structureless or granular eosinophilic membrane-like material adhering to the walls of non-collapsed or partially expanded alveoli, alveolar ducts, and terminal bronchioles. This membrane is composed of damaged and desquamated alveolar epithelial cells, fibrin, and a protein-rich matrix. No hyaline membranes are observed in {|###|}dead fetuses{|###|}. In cases of death within 8 hours after birth, the hyaline membranes are incompletely formed and mostly fragmented, floating freely in the alveolar spaces. The interstitial vessels of the alveoli show static blood.
bubble_chart Clinical ManifestationsMost of the affected infants are premature, with only about 5% being full-term. The maternal history often indicates anemia, prenatal uterine bleeding, cesarean section, breech delivery, multiple births, or pregnancy-induced hypertension syndrome, diabetes, and abnormal childbirth.
At birth, the heartbeat and breathing may appear completely normal. Generally, respiratory distress, cyanosis, expiratory grunting, and inspiratory retractions begin immediately after birth or gradually develop within 6 hours, progressively worsening. There is incoordination between thoracic and abdominal respiratory movements, with breathing transitioning from rapid to slow, irregular, or even apnea, accompanied by marked cyanosis. Although breathing may improve temporarily after emergency treatment, it often relapses, presenting as primary episodes that gradually intensify, with prolonged duration and shortened intervals between episodes. Body temperature is unstable and often fails to rise. Most deaths occur within 48 hours after birth. Some cases gradually improve with treatment, and if the condition persists beyond 72 hours, lung maturity increases, allowing most infants to recover progressively.
bubble_chart Auxiliary Examination
1. Amniotic fluid examination: Before birth, amniotic fluid is obtained via amniocentesis, or at birth by collecting the ruptured membrane's amniotic fluid for foam testing and the lecithin/sphingomyelin (L/S) ratio check.
Foam test: Equal amounts of amniotic fluid are placed into 5 small test tubes, to which varying amounts of pure alcohol are added. After vigorous shaking for 15 seconds and standing for 15 minutes, foam formation is observed. In cases of the disease, even a small amount of alcohol can prevent foam formation in the amniotic fluid.
Lecithin and sphingomyelin: In mature lung development, the lecithin (L) level in amniotic fluid reaches 3.5 mg/dl, and the lecithin/sphingomyelin ratio (L/S) should be 2–3:1. If L/S < 2:1, it indicates poor lung development.2. Gastric fluid oscillation test: 1 ml of gastric fluid is mixed with 1 ml of 95% alcohol, shaken for 15 seconds, and left to stand for 15 minutes. If a ring of foam persists along the tube wall, it is positive, preliminarily ruling out HMD; a negative result suggests the disease. The false-positive rate is only 1%, but the false-negative rate can reach 10%. The later the gastric fluid is sampled, the higher the false-negative rate, as amniotic fluid has already entered the intestines.
3. Amniotic fluid phosphatidylglycerol (PG) measurement: PG testing of aspirates from the pharynx or trachea after birth can provide early indications of the disease.
4. Blood tests: Blood pH, PaO2, and HCO3- decrease, while PCO2 and BE increase, showing metabolic acidosis. Blood potassium is often elevated early on but may decrease after diuresis during the convalescence stage.
5. Pulmonary X-ray examination: The severity of the condition can be classified into four grades.
Grade 1 shows fine millet-like ground-glass opacities with reduced lung translucency (Figure 1).

Grade 2 reveals millet-like shadows along with air bronchograms extending beyond the cardiac shadow.
Grade 3 includes the above findings with blurred cardiac and diaphragmatic borders (Figures 2, 3).

Grade 4 presents extensive white shadows termed "white lung," with black, bare-branched air bronchograms radiating from the hilum to the peripheral airways, forming "air bronchograms." High-pressure oxygen administration may improve X-ray findings (Figure 4).
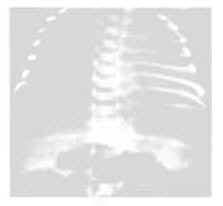
1. Medical history: Mostly premature labor, cesarean section infants, or those with a history of asphyxia, maternal diabetes, pregnancy-induced hypertension syndrome, etc.
Progressive dyspnea occurs within 6–12 hours after birth.
2. Signs: The infant appears dull, with a grayish or cyanotic complexion and flaccid limbs. Progressive dyspnea, expiratory moaning, and inspiratory retractions are observed. The heart rate initially increases and then slows, heart sounds transition from strong to weak, and a systolic murmur may be heard at the left sternal border. The respiratory rate ranges from 60–100 breaths per minute or higher, with irregular rhythm and intermittent pauses. Breath sounds are diminished in both lungs, and fine rales may be heard later, though early-stage lung rales are often indistinct. Dullness may be noted on percussion. The liver may enlarge.
3. Auxiliary examinations: The amniotic fluid foam test and gastric fluid oscillation test are both negative; the amniotic fluid lecithin/sphingomyelin (L/S) ratio is <2:1; blood pH, PaO2, and HCO3- are decreased, while PCO2 and BE are increased, indicating metabolic acidosis. Blood potassium is often elevated in the early stage but may decrease after diuresis during the stage of convalescence. Early chest X-rays show fine granular shadows in both lungs, eventually progressing to complete opacification ("white-out") with black "air bronchograms." X-ray examinations should be performed before positive-pressure ventilation, as recently collapsed alveoli may re-expand, resulting in a false-negative chest X-ray.
bubble_chart Treatment Measures
Whether clinically suspected or confirmed cases, active treatment should be implemented to strive to survive the first 3 days, as there is hope for survival.
I. General Treatment:
1. Maintain warmth, ensuring body temperature stays between 36–37°C, with an incubator relative humidity of around 50%. Use a monitor to track temperature, respiration, heart rate, and transcutaneous PO2, PCO2, and pH.
2. Regularly clear mucus from the pharynx to keep the airways unobstructed.
3. Ensure adequate nutrition and fluid intake. For those unable to breastfeed, administer 1/5 hypotonic sodium solution at 60–80 ml/(kg·d), increasing to 100–120 ml/(kg·d) from the second day onward via intravenous drip. For patients on mechanical ventilation, if the inhaled gas is already saturated with water vapor, reduce fluid intake to 50–60 ml/(kg·d).
4. Oxygen therapy and mechanical ventilation: Maintain PaO2 at 6.7–9.3 kPa (50–70 mmHg). Excessively high PaO2 can lead to retinopathy of prematurity (ROP) and blindness. An inspired oxygen concentration (FiO2) > 0.6 for over 24 hours may cause pulmonary toxicity, leading to bronchopulmonary dysplasia (chronic lung disease).
II. Surfactant (PS) Replacement Therapy:
Surfactants (PS) come in three types: natural, synthetic, and mixed formulations. Natural extracts from amniotic fluid, bovine, porcine, or ovine lung lavage are more effective than synthetic ones. Mixed formulations combine natural extracts with small amounts of synthetic dipalmitoyl phosphatidylcholine and phosphatidylglycerol.
Generally, administer PS at 100–200 mg/(kg·dose) suspended in 4 ml of saline. Deliver it via endotracheal tube into four different positions (supine, right lateral, left lateral, and supine again), followed by 1–2 minutes of mask-bag ventilation to ensure even distribution in both lungs. Improvement may be observed within 1–2 hours. Repeat the same dose every 12 hours. Multiple treatments (2–3 times) within the first 2 days can increase the cure rate to over 90%. Administering PS before the onset of normal breathing can serve as prevention.
III. Symptomatic Treatment:
1. Correct typical edema, electrolyte, and acid-base imbalances. For acidosis, prioritize 5% sodium bicarbonate at 3–5 ml/(kg·dose), or calculate based on measured BE and CO2-CP: BE × body weight (kg) = Na+ mmol (mEq), but do not exceed 6–8 mmHg/kg per day. For hypernatremia, use 0.3M THAM at 2–3 ml/dose intravenously. For hyperkalemia, administer 15% glucose at 50 mg/kg, with 1 IU regular insulin per 3–4 g glucose via IV drip.
2. Manage heart failure with rapid-acting digitalis preparations, such as strophanthin K at 0.01 mg/(kg·dose) or cedilanid at 0.015 mg/(kg·dose), given slowly intravenously. For patent ductus arteriosus, consider indomethacin at 0.02 mg/(kg·dose) for three doses at 12-hour intervals; for infants under 2 days old, halve the latter two doses.
3. For severe hypoxia-induced spasms, administer 20% mannitol at 5 ml/(kg·dose) intravenously.
4. In cases of respiratory failure, promptly use lobeline or nikethamide.
5. For dysphoria and spasms, administer diazepam at 0.2–0.3 mg/(kg·dose) intravenously, or phenobarbital sodium at 5–7 mg/(kg·dose) intramuscularly.
6. Closure of the ductus arteriosus. During mechanical ventilation or the convalescence stage after treatment, due to the relief of pulmonary arteriolar spasm, the pulmonary arterial pressure may decrease below the aortic pressure, resulting in left-to-right shunting. A large shunt volume can lead to heart failure and pulmonary edema, especially in infants weighing <1500g. Indomethacin can be administered intravenously to close the ductus arteriosus: for birth weight <1250g, 0.1mg/kg per dose; for those >7 days old, 0.2mg/kg, with additional doses at 12 and 36 hours. Oral administration of this drug is less effective. Direct infusion into the ductus arteriosus via cardiac catheterization yields better results. Surgical ligation should be considered if medication proves ineffective.
IV. Infection prevention and control: Strictly implement disinfection and isolation protocols; select effective antibiotics, typically penicillin at 20-250,000 U/(kg·d).
The mortality rate is very high, but most patients who receive early application of positive pressure ventilation can survive. Those who survive for more than 72 hours and do not have severe complications often produce sufficient surfactant, leading to gradual improvement in their condition. However, the prognosis is poor for those who develop intraventricular hemorrhage.
Strengthen prenatal care to prevent premature labor. Treat pregnant women with diabetes early, and delay cesarean section as much as possible until after the onset of childbirth. For pregnant women at risk of premature labor, with a negative amniotic fluid shake test, L/S < 2, or PG < 20 mg/L, and without severe hypertension or infection, oral betamethasone 0.5 mg or dexamethasone 0.75 mg can be administered three times daily for 2 days, starting 1–7 days before childbirth; alternatively, intravenous hydrocortisone 100 mg can be given every 12 hours for a total of 4 doses.
For premature infants at risk of this condition, especially those with no PG in endotracheal aspirates or a negative gastric shake test, one dose of surfactant (150 mg/kg) can be instilled, with a second dose administered 12 hours later if needed.
1. Group B β-hemolytic streptococcus (GBS) infection: Rarely seen domestically. Clinical manifestations and chest X-rays resemble HMD, but the infant often has a history of premature rupture of membranes or prolonged labor. The mother’s cervical swab culture is positive for GBS, and the infant’s gastric fluid or tracheal aspirate may reveal gram-positive cocci arranged in chains, with a positive urine streptococcal antigen test. If GBS infection cannot be ruled out, penicillin may be tried.
2. Wet lung: More common in full-term infants or cesarean-delivered infants. The condition is mild, the course is short, and the prognosis is good. The gastric fluid shake test is positive, and the chest X-ray does not show HMD. Lung qi swelling, pulmonary static blood, and interlobar effusion are more common, with occasional small amounts of pleural effusion.
3. Meconium aspiration pneumonia: More common in full-term infants and post-term infants, with a history of asphyxia and meconium aspiration. The gastric fluid shake test is positive, and the chest X-ray shows irregular patchy shadows with significant lung qi swelling.
4. Other differential diagnoses include congenital cardiopulmonary malformations, congenital metabolic diseases, primary atelectasis, pneumonia, pulmonary hemorrhage, pulmonary edema, pneumothorax, intracranial hemorrhage, diaphragmatic hernia, and severe anemia or polycythemia. Full-term infants should also be differentiated from adult-type RDS.




