| disease | Myocardial Infarction Complicated by Mitral Regurgitation |
Myocardial infarction involving the papillary muscles can lead to varying degrees of mitral regurgitation. Approximately 3% of patients with coronary atherosclerotic heart disease undergoing cardiac catheterization exhibit mitral regurgitation. Mitral regurgitation caused by coronary heart disease may result from acute or chronic papillary muscle ischemia. During myocardial infarction, the papillary muscles can completely rupture within hours due to acute ischemic necrosis. Although the chordae tendineae and valve leaflets show no pathological abnormalities, the corresponding mitral valve leaflets lose their opening and closing function, leading to severe mitral regurgitation shortly after the infarction. Among acute myocardial infarction cases, about 0.4–5% die from sudden-onset grade III mitral regurgitation due to papillary muscle rupture. In some patients, myocardial infarction causes ischemic necrosis of the papillary muscles but does not result in immediate complete rupture. Alternatively, due to prolonged ischemia, the necrotic myocardial tissue is gradually replaced by fibrous tissue, causing the papillary muscles to become thin, elongated, and weakened or lose their contractile function. Mitral regurgitation manifests more than two months after the myocardial infarction.
bubble_chart Pathological Changes
The apex of the anterior-lateral and posterior-medial papillary muscles each emits chordae tendineae, which connect to the edges of the mitral valve leaflets. Each papillary muscle is responsible for the chordae function of either the anterior or posterior half of the two mitral valve leaflets. During ventricular contraction, they tighten the mitral valve to prevent the leaflet edges from flipping into the left atrium, thereby avoiding insufficiency. The blood supply to the anterior-lateral papillary muscle comes from the diagonal branch of the left anterior descending artery and the marginal branch of the circumflex artery, while the posterior-medial papillary muscle is supplied solely by the posterior descending branch of the right coronary artery. Consequently, in coronary artery disease, the posterior-medial papillary muscle is more prone to ischemic changes than the anterior-lateral papillary muscle. Approximately 80% of acute papillary muscle ruptures occur in the posterior-medial papillary muscle. Papillary muscle necrosis and rupture due to myocardial infarction cause acute mitral valve insufficiency, leading to a significant backflow of blood from the left ventricle into the left atrium during ventricular contraction. This reduces left ventricular output, lowers blood pressure, and in severe cases, results in shock. Simultaneously, pulmonary vascular congestion occurs, leading to pulmonary edema.
In cases where acute myocardial infarction causes only partial rupture of the papillary muscle, the papillary muscle is stretched and elongated. During ventricular contraction, a portion of the mitral valve leaflet prolapses into the left atrium, resulting in mitral valve insufficiency. However, the degree of insufficiency is milder, with less regurgitation, and the impact on hemodynamics is relatively minor. In some cases, the ischemic papillary muscle does not rupture during myocardial infarction but is gradually replaced by fibrous scar tissue, losing its contractility. The weakened papillary muscle is stretched, and although the chordae tendineae remain connected to the papillary muscle, dysfunction of the papillary muscle allows the mitral valve leaflet to prolapse into the left atrium during ventricular contraction. Nonetheless, the mitral regurgitation is generally not severe, and the disease progresses slowly, though it can eventually lead to left heart failure. This scenario is more common among patients who undergo surgical treatment for mitral valve insufficiency more than two months after myocardial infarction.Cases of mitral valve insufficiency caused by papillary muscle rupture or dysfunction often involve myocardial infarction of the ventricular free wall. The extent of the infarction and the thickness of the affected myocardium vary widely, ranging from transmural infarction to infarction limited to the subendocardial region. In severe cases, mitral valve insufficiency due to papillary muscle rupture may coexist with ventricular septal rupture, ventricular free wall rupture, or ventricular aneurysm.
bubble_chart Clinical Manifestations
Echocardiography can demonstrate abnormal motion of the mitral valve leaflets, with failure of the anterior and posterior leaflets to coapt during ventricular systole, and can differentiate between papillary muscle rupture and papillary muscle dysfunction. In the former, during ventricular systole, the affected chordae tendineae and part of the mitral valve leaflets flip into the left atrium, with the anterior and posterior leaflets failing to coapt, and during ventricular diastole, they return to the left ventricle with blood flow. Sometimes, the distal segment of the ruptured papillary muscle attached to the chordae can be seen fluttering with the leaflets. In cases of papillary muscle dysfunction, reduced contractility of the papillary muscle is observed, with poor coaptation of the mitral valve leaflets during ventricular systole, and abnormal motion of the myocardial free wall is also evident.
Selective left ventriculography can confirm the diagnosis, assess the severity of mitral regurgitation, identify the location and extent of abnormal left ventricular wall motion, detect the presence of ventricular aneurysm, and exclude ventricular septal rupture. However, for critically ill patients, a cautious approach is advisable, and this examination should not be routinely performed.
Chronic mitral regurgitation caused by papillary muscle ischemia often presents with symptoms and signs of mitral regurgitation months after myocardial infarction. In the early stages, symptoms may be intermittent, and the severity of mitral regurgitation gradually worsens. The ventricles and left atrium become significantly enlarged, cardiac function declines, and heart failure develops.
bubble_chart Treatment Measures
In cases of complete papillary muscle rupture where surgical treatment is not promptly performed, approximately 75% of patients die from shock and heart failure within 24 hours of onset. Partial rupture has a better prognosis, with about 50% of cases surviving after one month and gradually progressing to chronic ischemic mitral regurgitation. Among coronary heart disease patients with mitral regurgitation, the 5-year survival rate is deficient 50%.
Surgical technique: For cases of complete papillary muscle rupture causing acute mitral regurgitation, the condition is severe and requires emergency surgical intervention. After diagnosis is confirmed via right heart Swan-Ganz catheterization, intra-aortic balloon pumping should be initiated immediately to temporarily improve or maintain circulatory function. Surgery is then performed under extracorporeal circulation combined with moderate hypothermia. A median sternotomy is performed, the sternum is split longitudinally, and the pericardium is opened to expose the heart. Simultaneously, a segment of the great saphenous vein is harvested for bypass grafting. Systemic heparinization is then administered, and cannulas are inserted into the superior and inferior vena cava or right atrium for venous drainage, while an arterial cannula is placed in the ascending aorta to initiate extracorporeal circulation. Cold saline is used for local deep cardiac cooling, an aortic cross-clamp is placed on the ascending aorta, and cold cardioplegic solution is injected via a needle inserted at the root of the ascending aorta. First, an end-to-side anastomosis is performed between the great saphenous vein and a coronary artery branch. Then, through a left atrial incision along the interatrial groove, the mitral valve is excised and replaced with a mechanical or biological prosthesis. Due to the fragile nature of the mitral annulus tissue, sutures should pass through sufficient tissue, and traction on the sutures should be gentle to avoid tissue tearing. Interrupted sutures with small pledgets can enhance suture security. After completing the mitral valve replacement, the aortic cross-clamp is removed to restore coronary blood flow. The aortic wall is then partially clamped to perform the anastomosis between the great saphenous vein and the ascending aorta. Performing the distal bypass graft anastomosis before valve replacement avoids the risk of left ventricular myocardial rupture in the infarcted area caused by the prosthetic valve when the heart is manipulated to expose the coronary artery branches.
The surgical approach for chronic ischemic mitral regurgitation depends on the pathology. First, a great saphenous vein-to-coronary artery branch anastomosis is performed, followed by addressing the mitral valve, usually via a left atrial incision along the interatrial groove. If the valve pathology is limited to the posterior leaflet, mitral valve repair and/or annuloplasty may be performed. For more extensive involvement of the anterior leaflet, valve replacement is necessary. In cases complicated by ventricular aneurysm and ventricular septal perforation, the aneurysm is resected through a left ventricular incision, the septal defect is closed, and after excising the affected papillary muscle and mitral valve, valve replacement is performed (Figure 1).
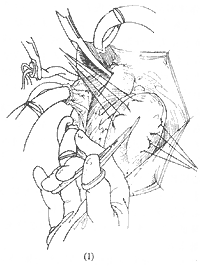
Figure 1 Surgical Treatment of Ventricular Aneurysm, Ventricular Septal Perforation, and Ischemic Mitral Regurgitation
(1) Incision of the ventricular aneurysm; (2) Resection of the aneurysm wall and suturing of the ventricular septal rupture; (3) Reinforcement of the ventricular septal suture with a patch; (4) Excision of the mitral valve and papillary muscles; (5) Suturing of the prosthetic mitral valve; (6) Completion of mitral valve replacement; (7) Closure of the left ventricular incision
Surgical Outcomes: The operative mortality rate is closely related to the extent of myocardial infarction, left ventricular function, and the timing of surgery. For patients undergoing surgical treatment within 1 week after myocardial infarction, the operative mortality rate is 40%; for those undergoing surgery 2–3 weeks later, it decreases to below 30%. The early mortality rate for surgical treatment of chronic ischemic mitral regurgitation is 10–15%. Factors influencing operative mortality include functional class, left ventricular ejection fraction, age at surgery, and the presence of a ventricular aneurysm. The 3-year survival rate postoperatively is approximately 50–65%, with most deaths occurring within the first year after surgery.





