| disease | Cardiac Myxoma |
Cardiac myxoma is the most common primary true tumor within the heart chambers. It is generally considered benign, with some complex manifestations and malignant tendencies, but some also regard it as a true tumor with a low degree of malignancy.
bubble_chart Pathological Changes
Whether it is a simple or complex cardiac myxoma, the pathological morphology of the tumor itself is not different.
(1) Gross observation
Tumor shape and color: The tumor shape is diverse and highly inconsistent, which can be spherical, oval, irregular, often with varying degrees of notches, or lobulated, cauliflower-like, finger citron-like, mature grape-like, or polyp-like. Polyp-like tumors float with pseudopods when placed in water. The basic color is light yellow, with scattered or patchy dark areas, and the degree of darkness varies greatly among individuals. Due to bleeding and its duration, it can appear bright red, deep red, dark red, light purple, or black-purple.
Tumor nature: The surface is smooth, translucent, jelly-like, soft to the touch, with a sense of tension or mucus. Calcified areas feel hard. The texture is extremely brittle and easily broken, and it can partially or completely detach.
Size and weight: There is a great individual variation, ranging from a few millimeters to tens of centimeters, and from a few grams to hundreds of grams. The heaviest in our country reached 500g.
Occupancy of the heart chamber: It can occupy a small part, most of it, or completely block the entire heart chamber, with blood passing only through the gaps.
Tumor stalk: Most commonly attached to the left atrial septum at the oval fossa (typical location), it can also be attached nearby or to the heart wall, the base of the atrioventricular valve, or extensively attached to any part of the septum or heart wall. The length and thickness of the stalk vary greatly, ranging from a few centimeters, a few millimeters, or no stalk with the tumor directly attached to the heart tissue. The cross-sectional area of its base can be only a few square millimeters, a few square centimeters, or diffusely adherent without a stalk.
The mobility of the tumor within the heart chamber depends on the length of the stalk and whether the tumor is adherent, the degree and extent of adhesion. A long stalk allows for greater movement with the cardiac cycle, while a short stalk results in less movement, and heavy adhesion may result in no movement.(2) Histological observation
Although cardiac myxomas can be detected clinically using various imaging techniques, the definitive diagnosis still relies on pathological and histological examination. Electron microscopy is not yet a routine examination item.
The typical histological image of a cardiac myxoma shows tumor cells in irregular or star shapes, often surrounded by halos, scattered or in small groups, with loose stroma rich in alcian blue-positive material. Tumor cells tend to distribute around small blood vessels, and a few myxomas may also show glands, bone trabeculae, etc. The stalk and heart wall are separated by elastic fibers, either dense or loose, with blood vessels from the heart wall supplying the tumor passing through, so the elastic fiber layer between the heart wall and the stalk can serve as a marker for complete tumor removal and infiltration. The muscular layer of the stirred pulse in the stalk and near the stalk heart wall may show myxoid changes. Some well-differentiated myxomas have the ability to implant, so the judgment of the benign or malignant nature of a myxoma depends not only on the degree of differentiation but also on its biological behavior such as infiltration and distant implantation.
Key points for histological differential diagnosis:
1. Thrombus: There is a theory that myxomas originate from thrombi, especially spherical thrombi in the atrium. However, myxomas and thrombi are ultimately two different types of pathologies. The surface of a thrombus is not covered by cells, and its organization process starts from the attached wall, whereas myxomas are antagonistic, with their non-degenerative surfaces covered by cells, and tissue necrosis and fibrinoid changes occur at the periphery, with the myxoma tissue structure always present near the stalk.
3. Recurrent and Recurrent Myxomas The gross and histological morphology of recurrent and recurrent myxomas of the heart are the same as those of sporadic myxomas. Recurrent myxomas have histological connections with the original myxoma, such as incomplete removal of the original myxoma, whereas recurrent myxomas have no histological connection with the original myxoma.
(3) Immunohistochemical Examination
As part of the etiological exploration, it has been found that all patients with familial cardiac myxoma have chromosomal abnormalities (haploidy) in their cells, indicating abnormal intracellular deoxyribonucleic acid (DNA) content. In contrast, only about 20% of patients with non-familial sporadic cardiac myxoma exhibit such changes (McCarthy 1989).
bubble_chart Clinical Manifestations
1. Classification of Cardiac Myxoma:
With the increase in the number of cases, accumulation of data, and advancements in research methods both domestically and internationally, the understanding of cardiac myxoma has gradually deepened. It can no longer be simply regarded as just a benign tumor. Based on the literature, cardiac myxoma can be classified into two major categories.
1. Simple or sporadic cardiac myxoma: This type of myxoma constitutes the vast majority of cases, mostly solitary, and often grows in typical locations (the area corresponding to the fossa ovalis of the interatrial septum in the left atrium), or multiple (about 20-4% of cases). Patients do not have myxomatous changes in other parts of the body, and the condition may not recur after a single routine elective surgical removal, with the heart and other parts of the body returning to normal or near-normal.
Myxoma syndrome can have seven pathological manifestations: ① Intracardiac myxoma; ② Cutaneous myxoma; ③ Myxoid fibroadenoma of the breast; ④ Skin spot pigmentation (including freckles and certain moles); ⑤ Primary pigmented nodular adrenocortical disease causing Cushing's syndrome; ⑥ Pituitary adenoma; ⑦ Testicular tumors, especially large cell calcifying Sertoli cell tumors. Patients with myxoma syndrome have one or several of these pathological changes outside the heart, with multicentric occurrence and a genetic tendency (35%).
Multicentric cardiac myxoma manifests as multiple simultaneous or metachronous (sequential recurrence) myxomas within the heart. Metachronous multiple myxomas may recur at the original site (nearby), in other parts of the same heart chamber, or in other heart chambers after a single surgical removal (not due to incomplete removal) and may recur multiple times.
2. Clinical Manifestations:
Cardiac myxoma is an intracardiac space-occupying lesion, and the pathophysiological changes and clinical manifestations it causes vary greatly among individuals due to differences in the heart chamber it occupies, the size of the tumor, its shape, growth rate, presence of adhesions, mobility, solitary or multiple, lobulated or not, presence of fragment shedding, hemorrhage, degeneration, and necrosis within the tumor, and the presence and severity of systemic autoimmune reactions.
(1) Mechanical obstruction of intracardiac blood flow
Cardiac myxoma occupies a certain position in the heart. If the volume is still small, it may not obstruct blood flow. As the tumor gradually enlarges, its obstructive effect on blood flow will become increasingly apparent. If the tumor is huge and fills the heart chamber, blood can only flow through the gaps in the tumor tissue, causing severe hemodynamic disturbances. In typical cases, the tumor has a considerable volume, a stalk of a certain length, no adhesions, and relatively high mobility, often moving between the two heart chambers across the valve with the cardiac cycle.
1. Left heart myxoma: In the left atrium, the tumor moves toward the mitral valve orifice during diastole and prolapses into the left ventricle through the valve orifice, returning to the left atrium during systole. Therefore, left atrial myxoma obstructs the mitral valve orifice during diastole, mimicking true mitral stenosis, leading to varying degrees of pulmonary congestion and some of the most common subjective symptoms (flusteredness 97%, shortness of breath 96%) and signs (diastolic murmur), clinically similar to rheumatic mitral stenosis. However, the degree of pulmonary congestion is generally mild, often disproportionate to the more severe subjective symptoms and signs. If the tumor is too large and cannot fully return to the left atrium during systole, becoming stuck at the valve orifice (syncope, sudden death), or if part of the tumor is attached to the mitral annulus or valve leaflet, obstructing mitral valve movement and affecting its closure, it can lead to mitral regurgitation. This can manifest as mitral stenosis combined with regurgitation, resulting in a biphasic murmur.
Cardiac myxoma in the left ventricle can obstruct the left ventricular outflow tract or the aortic valve orifice during systole, manifesting as aortic valve stenosis.
The atria and ventricles also undergo secondary changes due to lesions at the valve orifices, but the degree of change is usually milder compared to true valve membrane diseases. Left atrial myxoma may show mild to grade II enlargement of the left atrium.
2. Right heart myxoma Right heart myxomas are more commonly adherent, with more severe obstruction and less mobility. In the right atrium, they can obstruct the tricuspid valve orifice during diastole and/or affect the movement of the valve leaflets, presenting as tricuspid stenosis or combined stenosis and insufficiency. If the tumor is near the vena cava orifice or a mid-right atrial tumor enlarges to the vena cava orifice, it can obstruct venous return, leading to corresponding congestive reactions such as hepatomegaly and lower limb edema.
3. Multiple myxomas Depending on the different heart chambers occupied by the tumors, the number of tumors, the size differences, and the range of mobility, the impact on blood flow varies. When both left and right heart chambers are involved, the situation becomes even more complex, with the overall manifestation being a combination of conditions in each heart chamber.
(二)Stirred pulse embolism
Cardiac myxomas have loose, fragile tissue that is prone to fragment shedding. Whether fragments shed from a myxoma is not related to the duration of the disease or the size of the tumor, but closely related to the shape and structure of the myxoma. Polypoid or grape-like myxomas, with their surface parts of varying sizes, are prone to fragment shedding and forming tumor emboli. Right heart myxoma emboli entering the pulmonary stirred pulse can cause pulmonary infarction. Systemic stirred pulse tumor emboli can cause embolisms in any part of the body, more commonly cerebral embolism, femoral stirred pulse embolism, renal stirred pulse embolism, mesenteric membrane embolism, etc. The signs of stirred pulse embolism vary greatly depending on the size of the tumor emboli (from a few millimeters to several centimeters) and the size of the stirred pulse being embolized (from tiny stirred pulse to large stirred pulse), with clinical manifestations ranging from mild to severe, such as unconsciousness, hemiplegia, acute abdomen, limb necrosis or Raynaud's phenomenon, or no obvious signs of embolism.
Cardiac myxoma cells have the ability to implant, and the stirred pulse inner membrane at the site of the tumor embolus can tightly adhere to the tumor cells, or the tumor cells can invade the middle or outer layer of the vessel wall to form local myxoma tumor disease foci.
The incidence of stirred pulse embolism in myxomas in our country is about 15%, lower than the 30-40% reported abroad.
(三)Myxoma (like) lesions in other parts of the body outside the heart
Can cause local and/or endocrine disorders and other changes.
(四)Autoimmune reactions
Systemic autoimmune reactions caused by changes such as hemorrhage, degeneration, and necrosis within the cardiac myxoma. In repeated Zabing cases, there are often blood abnormalities (low antithrombin ATⅢ, heparin resistance, high platelet count, fast erythrocyte sedimentation rate up to 140mm/h, anemia, abnormal plasma proteins, electrophoretic changes, etc.), high fever (up to 40℃), urticaria, reduced appetite, weight loss, systemic failure, and other manifestations.
1. Medical History: Age (found from infants to the elderly), duration of illness (from a few days to over 20 years), symptoms, etc., can be used as references but are not specific enough to serve as a basis for definitive diagnosis. Compared to wind-dampness valvular disease (especially mitral valve disease), cardiac myxoma in adults has a shorter onset time, and the proportion of elderly cases is larger. Therefore, for elderly patients suspected of valvular disease with a short onset time, rapid progression, or sudden onset, the possibility of cardiac myxoma should be alerted. For children or young adults suspected of having right heart obstructive congenital heart disease, differentiation from cardiac myxoma should also be considered. Symptoms that appear or disappear with changes in body position can be highly suspected as cardiac myxoma and should be noted.
2. Signs: Similar to wind-dampness valvular disease or certain congenital heart diseases, they are also insufficient as a basis for definitive diagnosis. Murmurs that change in intensity, appear, or disappear with changes in body position were once considered characteristic signs of cardiac myxoma. Although they have strong diagnostic significance, their occurrence rate is low (about 1/3). If positional murmurs are not elicited, it is also insufficient to rule out cardiac myxoma. Tumor plop sounds strongly suggest left atrial myxoma.
3. Electrocardiogram (ECG): Not a diagnostic basis, although various changes such as right bundle branch block, first-degree atrioventricular block, premature contractions, atrial fibrillation, atrial enlargement, ST or T wave changes, ventricular hypertension, ventricular hypertrophy, etc., may be present.
4. Chest X-ray: May show pulmonary congestion and certain changes in heart morphology. If pulmonary congestion and heart shadow changes are mild while symptoms are severe and signs are more obvious, it suggests the possibility of cardiac myxoma, but it can only serve as an important reference and cannot be used for definitive diagnosis.
5. Cardiac Catheterization: Can show changes in cardiopulmonary function but is not helpful for the diagnosis of cardiac myxoma. It is an invasive examination and carries the risk of tumor rupture, fragment detachment leading to embolism, especially left atrial puncture, which should be contraindicated.
6. Selective or Digital Subtraction Angiography: Also an invasive examination. Although it may show filling defects suggesting intracardiac space-occupying lesions, for more mobile intracardiac myxomas (e.g., left atrial), the generally speeded angiographic series may not provide very clear images, unlike the dynamic images of non-invasive echocardiography.
7. Radionuclide Blood Pool Scanning: A minimally invasive examination that can clearly show intracardiac tumor shadows but is not as non-invasive and convenient as echocardiography.
8. Computed Tomography (CT) and Magnetic Resonance Imaging (MRI): Both are non-invasive examinations and can clearly show intracardiac space-occupying lesions, but they are expensive and not suitable for routine examination of cardiac myxoma.
9. Laboratory Tests: Cardiac myxoma, especially in cases with severe systemic reactions, often presents with anemia (hemoglobin can be as low as 40-50g/L), increased erythrocyte sedimentation rate (ESR) (can be >120mm/h), changes in immunoglobulins IgM, IgG, IgA, etc., but these are not specific. These changes can only serve as references for understanding the systemic condition and cannot be used as a basis for definitive diagnosis.
In summary, for the diagnosis and differential diagnosis of cardiac myxoma, the possibility of this disease should be considered under the following circumstances:
⑴ Symptoms appear late, and the patient is older;
⑵ The course of the disease is short, and symptoms progress rapidly;
⑶ The patient was previously healthy but suddenly presents in a critical condition;
⑷ Symptoms can be induced or relieved by changes in body position;
⑸ Murmurs can appear, intensify, weaken, or disappear with changes in body position;
⑹ Presence of tumor plop sounds;
⑺ Chest X-ray shows insignificant pulmonary blood increase and minor heart shadow changes, while symptoms and signs are severe, showing a disproportionate relationship;
⑻ Long-term fever or prolonged high fever, increased ESR, anemia, changes in protein electrophoresis, etc., without signs of wind-dampness activity or infection, and internal medicine treatment is ineffective. {|117|}
⑼ Heart failure that is difficult to control over a long period of time;
10. Sinus rhythm, without obvious cause, sudden or recurrent stirred pulse embolism.
However, echocardiography is a powerful tool, being non-invasive, simple, safe, reliable, and can be repeated multiple times as an effective method.
10. Echocardiographic diagnosis of cardiac myxoma. M-mode ultrasound can provide a qualitative diagnosis, but two-dimensional echocardiography is the preferred method for quantitative diagnosis, reflecting the following characteristics: the morphology and contour of the tumor; the size of the tumor; distinguishing between localized and diffuse tumors; whether the echo at the tumor margin is clear, and whether there is a membrane echo; differentiating between intracardiac, myocardial, cardiac wall, and extracardiac tumors; whether the involvement is single or multiple cardiac chambers; displaying the attachment site, length, or other forms of origin of the stalk; the degree of morphological variation during tumor movement; the number of tumors; the degree of tumor echo and distribution characteristics; secondary changes including cardiac enlargement and deformation, valve membrane dysfunction, pericardial effusion, etc.
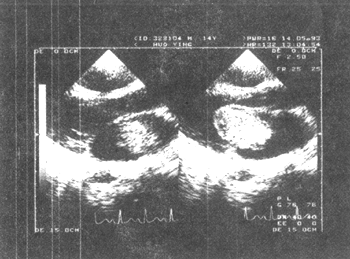
(1) Left atrial myxoma (Figure 1) Definitive diagnostic criteria: ① Abnormal spotty echoes are seen in the left atrium, clustered together. The contour is clear, the edges are relatively regular, roughly elliptical or long elliptical in shape, with uniform internal echo intensity, and the base is on the interatrial septum. If the stalk is large, the location and size of the stalk can be displayed (such as the long-axis view of the left ventricle at the left sternal border, the long-axis view of the inflow tract of the left and right ventricles, the apical four-chamber view, and the short-axis view of the large stirred pulse). ② The clustered echoes of the tumor move between the left atrium and left ventricle with the cardiac cycle. During systole, the entire tumor can return to the left atrial cavity, and during diastole, it reaches the mitral valve or passes through the mitral valve orifice into the left ventricle. Its position in the left atrium and left ventricle varies depending on the attachment site of the stalk and the size and shape of the tumor (long-axis view of the left ventricle at the left sternal border, long-axis view of the inflow tract of the left and right ventricles, apical four-chamber view). ③ On the short-axis view at the mitral valve level of the left ventricle, the tumor echo can be seen behind the mitral valve during systole, separable from the mitral valve, and retracting into the left atrium. During diastole, the tumor moves with the blood flow, prolapsing through the mitral valve orifice into the left ventricle, and the circular mitral valve orifice can be filled with tumor echoes.
|
| |
| Systole | Diastole |
Figure 1 Echocardiogram of left atrial myxoma (long-axis view of the left heart)
(2) Right atrial myxoma: The findings are similar to those of left atrial myxoma. The abnormal echo cluster of the myxoma is in the right cardiac chamber, in the right atrium during systole, and moves towards the right ventricle with the tricuspid valve during diastole, reaching the tricuspid valve orifice or passing through the tricuspid valve orifice into the right ventricular cavity, even protruding into the right ventricular outflow tract (clear tumor echo images can be obtained by subcostal exploration, and the long-axis view of the inferior vena cava can observe the right atrial myxoma located at the entrance of the inferior vena cava).
(3) Ventricular myxoma: During systole, the abnormal echo cluster protrudes into the left ventricular outflow tract or right ventricular outflow tract, and during diastole, it is in the left ventricular cavity or right ventricular cavity, showing the abnormal echo cluster swinging regularly with the blood flow direction in the ventricular cavity.
⑷ Cardiac multiple myxomas: Most commonly found in the left atrium, followed by the right atrium, less frequently in the left ventricle, and least in the right ventricle. Two-dimensional ultrasound has a definitive diagnostic value. However, for multiple myxomas within the same cardiac chamber, ultrasound may not accurately estimate the number and size of the tumors. It is necessary to change positions and conduct multiple scans to avoid missing small and less mobile lesions such as fistula disease.
bubble_chart Treatment Measures
(1) Surgical Indications and Timing
Once a cardiac myxoma is diagnosed, it must be actively managed, and surgical removal should be arranged as early as possible without exception. Since patients with cardiac myxoma are often threatened by stirred pulse embolism and/or sudden death, it is crucial to act promptly to alleviate these risks.
1. For patients with simple cardiac myxoma without systemic reactions, routine elective surgery can be considered, but it must be prioritized and not delayed.
2. For those with severe systemic reactions, rapid disease progression, and dangerous signs, emergency surgery should be arranged after excluding non-myxoma factors.
3. Emergency surgery should be arranged for patients with recurrent stirred pulse embolism that poses a death threat.
4. For patients with prolonged fever, ineffective antibiotic treatment over a period, and where high fever cannot be ruled out as being caused by the myxoma itself, surgery should be performed urgently while continuing antibiotic treatment, without delay.
5. For cases showing chronic heart failure, physical weakness, inability to lie flat at night, orthopnea, hepatomegaly, ascites, and lower limb edema, surgery should be arranged after ruling out other factors and actively controlling heart failure, once the condition stabilizes.
(2) Surgical Contraindications
There are no surgical contraindications for cardiac myxoma itself, but the following conditions should not be treated with myxoma surgery alone.
1. Cachexia caused by malignant tumors other than cardiac myxoma, where the latter cannot be surgically removed at the same time.
2. Disseminated subcutaneous node infection not yet under control.
3. Terminal cases of cardiac myxoma, in a near-death state, unable to withstand the burden of open-heart surgery under extracorporeal circulation (must wait until the rescue condition stabilizes).
4. Severe purulent infection foci in the body that have not been cleared and controlled.
5. Concurrent complex congenital heart and vascular malformations and/or pulmonary organic hypertension that cannot be corrected by conventional surgical methods (consider heart or cardiopulmonary transplantation).
Grade I heart failure (fast heart rate), anemia, fast erythrocyte sedimentation rate, and high fever, if not caused by other factors but by the cardiac myxoma itself, are not contraindications for myxoma removal. Existing stirred pulse embolism and its sequelae, as well as concurrent endocarditis, are also not contraindications for cardiac myxoma surgery.
(3) Preoperative Preparation
For simple cases, in addition to the general requirements for elective surgery, patients should be given appropriate rest, avoid excessive activity, and avoid sudden body movements.
Emergency surgery should follow emergency requirements.
(4) Key Points of Surgical Treatment
1. During the entire process of transporting the patient to the operating room until the anesthesia and positioning, avoid excessive and sudden movements of the patient's body.
2. Anesthesia should be managed as for critically ill patients, paying attention to heparin resistance. During extracorporeal circulation, microporous (40µ) filters should be installed at both arterial and venous ends to prevent microemboli from entering the body. When the surgical field is fully suctioned by an external suction device, pay attention to the oxygenator fluid level and replenish it in time if necessary.
3. Prevent tumor emboli formation. Throughout the surgery, always be vigilant for tumor fragments breaking off and forming emboli. For example: ① Avoid severe vibrations when sawing the sternum through a median incision; ② Before blocking the circulation, avoid moving or palpating the heart, and do not perform finger intracardiac exploration; ③ Perform surgical operations gently; ④ After tumor removal, thoroughly rinse and suction the heart chambers; ⑤ Install microporous filters at both the stirred pulse and venous ends of the heart-lung machine.
4. Choose an appropriate heart incision to fully expose the tumor (stalk). Different heart incisions should be used for myxomas in different heart chambers, very large tumors, tumors in special locations (stalks), or multiple myxomas, with a large enough incision to fully expose the tumor and its stalk.
5. Complete resection, remove the tumor intact. Remove part of the normal myocardium along with the tumor, take out the tumor completely, leaving no residue.
6. Pay attention to protecting the myocardium, maintaining normal heart function, and ensuring smooth recovery.
7. Correct blood abnormalities. Cardiac myxomas often present with blood abnormalities (anemia, acid-base imbalance, electrolyte disturbances, heparin resistance, etc.), which should be adjusted during surgery to achieve or approach a normal state, ensuring a smooth postoperative recovery.
(V) Key Surgical Techniques and Prevention of Major Complications
1. Cannulation
⑴ Right atrial tumor: The venous cannula should be inserted as close as possible to the atrial wall at the entrance of the vena cava; if the tumor is large and suspected to invade the vena cava orifice, the superior and inferior vena cava (including dissection of the diaphragm) should be dissected and the cannula inserted directly from the venous wall; if a giant myxoma completely fills the right atrial cavity and the proximal part of the vena cava, making cannulation from the vena cava wall impossible, cannulation should be performed from the external iliac vein for deep hypothermia (<20°C) circulatory arrest. After the tumor is removed from the right atrium, the cannula is repositioned in the right atrium for rewarming.
⑵ Left atrial tumor: It is not necessary to insert a left atrial decompression drainage tube in advance to avoid disturbing the tumor. Instead, a small incision can be made directly in the left atrial wall near the right superior pulmonary vein to drain left atrial blood into the pericardial cavity, using a low-pressure suction tube to draw blood from the pericardial cavity back into the oxygenator. After the myxoma is removed, a left atrial tube is inserted through this small incision for drainage and left atrial monitoring.
2. Cardiac Incision
⑴ Right atrial tumor and general left atrial tumor: An incision in the anterior wall of the right atrium (for right atrial tumor) and an additional atrial septal incision (for left atrial tumor) facilitate both the removal of the myxoma and the exploration of the cardiac chambers.
⑵ For giant left atrial tumors or deeply located tumors in the posterior wall of the left atrium, a combined left and right atrial incision can be used, extending the incision from the anterior wall of the right atrium and the atrial septum to the lateral wall of the left atrium.
⑶ Tumors near the tricuspid valve orifice in the right ventricle can be removed through a right atrial incision; tumors in the right ventricular outflow tract, near the apex, or with extensive adhesions require removal through an anterior wall incision of the right ventricle.
⑷ Tumors in the left ventricular outflow tract with a long stalk can be removed through an incision at the root of the aorta. If the tumor is near the left ventricular apex, an incision parallel to the left anterior descending artery in the avascular area of the left ventricular apex should be made. After tumor removal and cleaning, the left ventricular incision is sutured using a sandwich technique with pledget strips.
3. Tumor Removal
⑴ Avoid directly clamping or touching the tumor, especially grape-like or polypoid myxomas, to prevent fragmentation and detachment.
⑵ Identify the attachment site of the stalk, suture the stalk and myocardial tissue with traction sutures, or grasp and lift it with a rat-tooth forceps. Use a right-angle forceps to probe the size and extent of the stalk, and remove the surrounding myocardial tissue (atrial septum, full thickness of the atrial wall, ventricular septum, and a sufficient thickness of the ventricular wall) in a concentric circle slightly larger (0.5-1 cm) than the stalk. Simultaneously, enlarge the cardiac incision to completely remove the tumor.
⑶ If the tumor is large, heavy, or fragile and prone to fragmentation (grape-like, polypoid), the cardiac incision should be larger, and a suitably sized, bendable spoon or a low-suction aspirator can be used to support or hold the tumor for removal.
⑷ If the tumor is extensively adherent to the myocardium or valve membrane, it should be completely removed without compromise.
⑸ For giant intracardiac myxomas with extensive adhesions that cannot be removed through a conventional incision, consider autologous orthotopic heart transplantation: remove the heart, excise the tumor, repair the defect, and reposition the heart.
4. Post-Tumor Removal Management
⑴ Check if the heart tumor has been completely removed.
⑵ Inspect the removed tumor to ensure it is intact.
⑶ Use a high-pressure suction device to thoroughly remove residual tumor fragments from the cardiac chambers and pulmonary veins, followed by thorough rinsing with saline.
⑷ Carefully repair the myocardial tissue defect.
⑸ Directly suture or patch the septal or wall defect.
⑹ If the valve membrane defect cannot be repaired, replace it with a prosthetic valve.
⑺ If the main conduction system is damaged, install a permanent pacemaker.
5. Prevention of Myxoma Fragment Embolism
⑴ For right heart tumors, clamp the pulmonary artery before making the cardiac incision to prevent fragments from entering the pulmonary artery.
⑵ Thoroughly rinse and suction the heart after tumor removal.
⑶ Before releasing the aortic clamp, fully expel blood from the aorta to flush out microemboli.
6. Prevention of Postoperative Low Cardiac Output Syndrome (especially with left ventricular incision)
⑴ Improve cardiac function preoperatively.
⑵ Avoid unnecessary excessive or large incision injuries.
(3) Fully implement myocardial protection.
⑷ Maintain electrolytes (potassium, magnesium, calcium), blood volume, and blood quality within normal ranges.
(6) Postoperative Management
The general postoperative management of cardiac myxoma is similar to that of other cardiac surgeries, with special attention to signs of tumor embolization. In case of limb embolism, active thrombectomy should be performed, and for cerebral embolism, active symptomatic and supportive treatment should be provided.
The management of low cardiac output syndrome is also similar to that of other cardiac surgeries, which includes replenishing blood volume, using drugs to strengthen the heart, diuresis, and adjusting blood pressure. If necessary, intra-aortic balloon pump or left/right heart assist circulation should be performed early. For arrhythmias, electrolyte disturbances should be corrected, appropriate antiarrhythmic drugs should be used, and temporary or permanent pacemakers should be installed.
(7) Postoperative Outcomes and Follow-up Requirements
It is particularly important to provide discharge guidance to cardiac myxoma patients to improve their ability to self-assess their condition, ensure follow-up requirements, and strive for early detection of recurrence or relapse.
Some follow-up data from China with more than ten years indicate that postoperative recurrence cases account for about 1-2%, while foreign literature estimates it at 5%. The threat of postoperative recurrence or relapse makes long-term (lifelong) regular follow-up very necessary.
The follow-up content, in addition to self-perception (symptoms) and signs, mainly includes echocardiography. It is required to be performed once every six months within 4 years after surgery, and once a year after 4 years.
Follow-up methods: If conditions permit, return to the operating hospital for outpatient review, or review at a local hospital and notify the operating hospital by communication.