| disease | Rheumatic Mitral Insufficiency |
The most common lesion caused by wind-dampness heat invading the mitral valve is fibrosis and thickening of the valve membrane, fusion of the commissures, and narrowing of the valve orifice, resulting in pure mitral stenosis. About one-third of cases involve mitral stenosis combined with insufficiency. Pure mitral insufficiency is relatively rare, accounting for only about 5% of wind-dampness-related mitral valve lesions. In adults, the causes of mitral insufficiency, apart from wind-dampness-related valve membrane disease, also include coronary atherosclerosis leading to papillary muscle infarction and rupture; mitral insufficiency occurring secondary to left ventricular enlargement caused by aortic valve stenosis or insufficiency; mucoid degeneration and thickening of the mitral valve leaflets, elongation of the leaflets causing prolapse and resulting in insufficiency; bacterial endocarditis complicating mitral valve lesions leading to mitral insufficiency; and chest trauma causing mitral insufficiency, which is extremely rare. Mitral commissurotomy, especially closed procedures, can result in leaflet tearing or chordae rupture, leading to traumatic or iatrogenic mitral insufficiency.
bubble_chart Pathological Changes
A case of wind-dampness-induced mitral stenosis with insufficiency is caused by prolonged and recurrent inflammation of the mitral valve membrane due to wind-dampness heat. The mitral valve membrane becomes fibrotic, thickened, and rigid, with fused commissures, resulting in stenosis of the valve orifice. Simultaneously, the valve leaflets become deformed due to fibrotic contracture, and the free edges of the valve orifice become thickened or calcified, curling unevenly. This prevents the anterior and posterior leaflets from closing properly during ventricular systole. The chordae tendineae and papillary muscles also become fibrotic and shortened, pulling the leaflets toward the ventricular cavity, thereby restricting leaflet mobility and impairing the valve's opening and closing function. As a result, the mitral valve exhibits both stenosis and insufficiency.
In cases of isolated mitral insufficiency, although the valve membrane shows some degree of fibrosis and thickening, the commissures of the leaflets remain unfused, allowing unobstructed blood flow through the mitral orifice. The primary pathology is the dilation of the mitral annulus, which is caused by acute wind-dampness myocarditis leading to left ventricular enlargement. The mitral annulus thickens as the left ventricle expands, with the posterior leaflet's annular base showing more pronounced dilation, resulting in relative insufficiency of the leaflet area and failure of the valve orifice to close during systole. If the wind-dampness heat is properly treated during the acute phase and the myocarditis resolves, the left ventricle and annulus may shrink and return to normal, eliminating the insufficiency. However, if the myocarditis is left untreated or treatment fails, the left ventricle and annulus continue to enlarge. Over several years, mitral insufficiency further exacerbates left ventricular and annular dilation, worsening the degree of insufficiency. During systole, the mitral leaflets fail to coapt, increasing tension on the chordae tendineae, which may rupture. The leaflets may also exhibit fibromyxoid degenerative changes due to trauma from the impact of systolic blood flow in the left ventricle.In cases of mitral insufficiency, the left ventricle, left atrium, and mitral annulus are all significantly enlarged. Chronic regurgitation during left ventricular systole gradually stretches the left atrial wall, thinning it over time and greatly increasing the left atrial volume. During diastole, blood from the left atrium still flows smoothly into the left ventricle, causing a rapid drop in left atrial pressure. Consequently, pulmonary circulation pressure does not rise significantly, and complications such as pulmonary hypertension or pulmonary edema are rare or develop slowly. In cases of mitral insufficiency caused by myocardial infarction-induced chordae or papillary muscle rupture or traumatic chest injury, the sudden onset prevents the left atrium from adapting to the abrupt increase in regurgitant volume. Left atrial pressure rises rapidly, elevating pulmonary vascular pressure and leading to pulmonary edema and hypertension. Occasionally, pulmonary artery pressure may approach systemic levels, but it can normalize after correcting the mitral insufficiency.
In mitral insufficiency cases, during diastole, the left ventricle receives both blood returning from the pulmonary veins to the left atrium and blood regurgitated into the left atrium during the previous heartbeat. Prolonged diastole and increased filling volume gradually lead to left ventricular dilation and hypertrophy. In the advanced stage, pulmonary congestion and elevated pulmonary circulation pressure may cause right heart failure. During systole, part of the left ventricular blood regurgitates into the left atrium, reducing the effective blood flow into the systemic circulation.bubble_chart Clinical Manifestations
The clinical manifestations of mitral regurgitation vary in severity, depending on the acuity of onset, stage of disease progression, volume of regurgitation, and functional status of the left ventricle.
Grade I mitral regurgitation is mostly asymptomatic, with only a heart murmur detected during physical examination.
In cases with a prolonged course and significant regurgitation, despite an increased left ventricular stroke volume, the effective systemic blood flow is reduced, leading to symptoms such as lack of strength, fatigue, reduced exercise tolerance, and dyspnea upon exertion. However, resting dyspnea and orthopnea are rare. Advanced-stage mitral regurgitation may present with symptoms of left and right heart failure, though acute pulmonary edema, hemoptysis, or systemic embolism are far less common compared to mitral stenosis. Atrial fibrillation is also less frequent than in mitral stenosis.
In cases of acute mitral regurgitation caused by coronary atherosclerotic heart disease, chest trauma, or closed mitral commissurotomy, the left atrium cannot adapt to the abrupt hemodynamic changes. Consequently, the pressures in the left atrium and pulmonary veins rise, transmitting to the pulmonary capillaries, arterioles, and arteries, increasing right ventricular afterload and pulmonary blood volume, leading to pulmonary congestion and potentially pulmonary edema. Clinically, this manifests as severe dyspnea, orthopnea, and symptoms of right heart failure.
Physical examination: In grade I mitral regurgitation, aside from a systolic murmur at the apex, no other abnormal signs may be present. In moderate or severe regurgitation, a stronger, diffuse precordial impulse may be palpated, with the apical impulse displaced downward and to the left. A rough, loud (grade 3 or higher), prolonged holosystolic murmur is audible at the apex, weakening during deep inspiration and slightly intensifying during expiration, often radiating to the midaxillary line. The direction of radiation correlates with the lesion site. If the posterior leaflet is primarily affected, the murmur may radiate to the sternum or aortic area; if the anterior leaflet is involved, the regurgitant jet strikes the posterior left atrial wall, and the murmur may radiate to the spine or vertex. Some cases may exhibit a systolic thrill. Occasionally, a short diastolic rumbling murmur due to increased flow across the mitral valve may be heard at the apex. The first heart sound is diminished or obscured by the murmur. The second heart sound at the pulmonary area is normal or slightly accentuated, with splitting due to premature aortic valve closure. A third heart sound may be audible at the apex. The pulse may be normal or bounding. In advanced stages, signs of right heart failure such as jugular vein distension, hepatomegaly, and lower limb edema may appear. In combined mitral stenosis and regurgitation, both a prolonged diastolic rumbling murmur and a holosystolic murmur are audible at the apex, with a louder first heart sound.The progression and prognosis of mitral regurgitation depend on the age of onset and underlying etiology, with left ventricular function being a critical prognostic factor.
Rheumatic mitral regurgitation typically progresses slowly. Patients with good left ventricular compensation may remain asymptomatic for years after the detection of a heart murmur. The onset of clinical symptoms indicates declining left ventricular compensation, progressive ventricular dilation, and rapid deterioration.
The course of mitral regurgitation due to mitral valve prolapse resembles that of rheumatic mitral regurgitation.
In cases of acute mitral regurgitation caused by myocardial infarction, chest trauma, infective endocarditis, or iatrogenic injury during mitral stenosis surgery, the onset is abrupt, with rapid deterioration and potential death from acute left ventricular failure and pulmonary edema within a short period.
bubble_chart Auxiliary Examination
Chest X-ray examination: The chest X-ray shows enlargement of the left atrium and left ventricle, with a double-density shadow formed at the right border of the heart. The pulmonary artery segment is prominent, and the aortic arch is small. Fluoroscopy of the chest X-ray reveals expansile pulsation of the left atrium during systole, with strong pulsation of the left ventricle. Barium esophagography shows the esophagus being displaced posteriorly by the enlarged left atrium. There are no significant changes in the pulmonary vascular markings, or they may show grade I dilation. The X-ray examination can also determine the presence of calcification in the valve annulus.
Electrocardiogram (ECG) examination: Grade I mitral regurgitation may not show abnormal ECG findings. Moderate or severe regurgitation and cases with a longer disease course may exhibit left ventricular hypertrophy, possibly accompanied by strain and left axis deviation. Cases with pulmonary hypertension may show signs of left and right ventricular hypertrophy. Long-standing cases often present with atrial fibrillation.
Cardiac catheterization and selective left ventriculography: The left atrial pressure is elevated, with a mean systolic pressure reaching 2–2.7 kPa (15–20 mmHg). The pressure curve shows a high and sharp V wave with a steep descent, where the V wave is larger than the Q wave. In cases of pure mitral regurgitation, the diastolic pressure difference between the left atrium and left ventricle is not significant. Pulmonary capillary wedge pressure and pulmonary vascular resistance may be elevated to varying degrees, and cardiac output may decrease. Selective left ventriculography shows contrast reflux into the left atrium during systole. The severity of mitral regurgitation can be estimated based on the amount and density of contrast reflux into the left atrium. In cases with significant reflux, the contrast may fill the entire left atrium with high density, while the amount of contrast entering the aorta is relatively reduced. For patients over 40 years old being considered for surgical treatment, selective coronary angiography should also be performed.
Echocardiography examination: Grade I mitral regurgitation may not show abnormal echocardiographic findings. As the severity of regurgitation progresses, the left ventricular volume load gradually increases, leading to enlargement of both the left ventricle and left atrium. Echocardiography reveals enhanced contractility of the interventricular septum and left ventricular posterior wall, and the left atrium may show expansile pulsation during systole. If the valve leaflets, chordae tendineae, and papillary muscles are thickened, the echo reflectivity increases, and incomplete coaptation of the mitral valve leaflets is observed during ventricular systole. In cases of mitral stenosis with regurgitation, the valve orifice appears small with incomplete coaptation. For mitral regurgitation caused by chordae tendineae rupture, echocardiography may show the ruptured valve leaflet flipping into the left atrium during systole and rapidly returning to the left ventricle during diastole. In cases of mitral annular calcification, calcified plaques or nodules may show increased echo reflectivity, and grade III calcification may present as large or crescent-shaped echo enhancement of the entire annulus.
bubble_chart Treatment Measures
Since 1951, Bailey, Nichols, Davila, Glover, and others have performed closed-heart surgeries, using pericardial patches sewn into tubular shapes, veins, or membranes to loosely place below the mitral valve orifice. During left ventricular contraction, these partially obstructed the valve orifice or passed through the anterior and posterior leaflets, partially suturing the orifice or tightening the atrioventricular ring externally. However, due to high surgical mortality and poor long-term efficacy, these methods were abandoned. In 1957, Lillehei and Merendino performed mitral annuloplasty under extracorporeal circulation to treat mitral regurgitation. In 1961, Starr first reported the successful replacement of the mitral valve with a caged-ball mechanical valve membrane. In 1968, Carpentier developed an elastic artificial atrioventricular ring to suture and reduce the enlarged mitral annulus for treating mitral regurgitation. Later, he improved mitral valve membrane plasty and repair, enhancing treatment outcomes.
**Surgical Indications:** Approximately half of the cases of rheumatic mitral regurgitation require valve membrane replacement or plasty and repair. Current diagnostic methods are still inadequate for accurately determining the appropriate treatment preoperatively. At present, neither mechanical valve membranes nor biological valve membranes are perfect, with high postoperative complication rates and unsatisfactory long-term outcomes. Therefore, for patients with mild clinical symptoms, functional class I–II, and no significant left ventricular enlargement on physical examination, chest X-ray, or echocardiography, surgery should be postponed, and regular follow-up examinations should be conducted to monitor disease progression. On the other hand, the progression rate of left ventricular dysfunction is difficult to predict. In some cases with functional class III or higher, the left ventricular myocardium often shows permanent interstitial fibrous scar lesions, increasing surgical risks and compromising long-term outcomes. Severe left atrial enlargement also adversely affects surgical results. Thus, the optimal time for surgery is when the left ventricle begins to show irreversible changes, even if clinical symptoms are not yet severe. Recent advancements in echocardiography now allow for serial measurements of left ventricular end-systolic volume, ejection fraction, and regional wall motion abnormalities, enabling early detection of left ventricular dysfunction and aiding in surgical timing decisions. For patients with functional class III or IV, even if the ejection fraction drops to 0.40, surgery can still improve hemodynamics, increase left ventricular output to the aorta, alleviate symptoms, and prevent or delay further left ventricular dysfunction.
For acute mitral regurgitation caused by iatrogenic or infective endocarditis and chordae tendineae rupture, surgery can be delayed if medical treatment controls pulmonary venous hypertension and endocarditis, with regular follow-up. If medical treatment fails, immediate mitral valve membrane plasty and repair are required. Patients with grade III pulmonary vascular obstructive disease, chronic right heart failure, or failed medical therapy are not suitable for surgery.
**Surgical Procedures:** Depending on the valve membrane pathology, the following options are available for mitral regurgitation: 1. Mitral annuloplasty or reconstruction. 2. Mitral valve membrane plasty and repair. 3. Mitral valve membrane replacement.
**(1) Mitral Annuloplasty or Reconstruction** The goal is to reduce the mitral annulus and improve leaflet coaptation. This is suitable for cases with mild valve and subvalvular pathology, good leaflet mobility, and pure mitral regurgitation primarily caused by annular dilation.
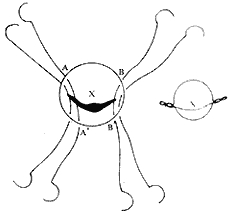
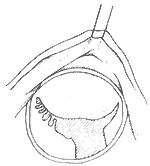
A median sternotomy is performed, and the sternum is split longitudinally. The anterior pericardium is incised in an "I" shape to expose the heart. A small left atrial incision is made along the interatrial groove to digitally explore the mitral valve for lesions, the site of regurgitation, and its grade I severity. A single large venous cannula is then inserted into the right atrium, or separate venous cannulas are placed into the superior and inferior vena cavae. An arterial cannula is inserted into the ascending aorta. After initiating cardiopulmonary bypass, systemic temperature is reduced to approximately 25°C, and cold saline is infused into the pericardial cavity to lower myocardial temperature. The ascending aorta is clamped, and cold cardioplegic solution is injected into its root. A longer left atrial incision along the interatrial groove is made to expose the mitral valve. In most cases, regurgitation is localized to the posteromedial commissure, where 2-0 sutures are placed in a figure-of-eight or pledgeted mattress fashion at the annulus of the posteromedial commissure (Figure 1). This shortens the annular length at the base of the posterior leaflet, allowing coaptation of the anterior and posterior leaflets during ventricular systole. If regurgitation is confined to the anterolateral commissure, the annular length at the base of the posterior leaflet in this region is similarly shortened. For cases with significant annular dilation and regurgitation along the entire valve orifice, pledgeted mattress or figure-of-eight sutures are placed at both the anterolateral and posteromedial commissures to reduce annular length. However, approximately 4 cm of annular length should be preserved at the base of the anterior leaflet, and 2–2.5 cm at the base of the posterior leaflet, leaving a residual valve orifice of at least 3 cm (accommodating two fingers) to avoid stenosis (Figure 2). When placing sutures at the anterolateral commissure, deep needle penetration should be avoided to prevent injury to the circumflex coronary artery. After tying the sutures, a venting catheter is inserted either through the left ventricular apex or a multi-hole cannula is placed in the ascending aorta. Saline is then injected under pressure into the left ventricular cavity to assess the adequacy of regurgitation correction.
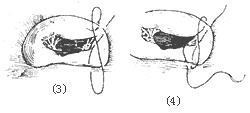
Figure 1 Mitral Annuloplasty
⑴ Post-reduction of the medial commissure ⑵ Reduction of both commissures
⑶, ⑷ demonstrate the "figure-of-eight" suturing technique for reduction
|
| |
| (1) | |
|
|
|
| (2) | (3) |
Figure 2 Limits of Annular Reduction
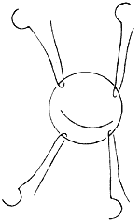
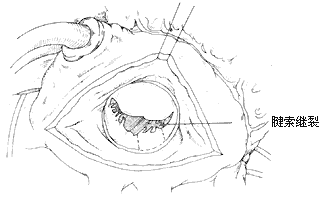
Mitral Annular Plication: Shore et al. reported in 1980 the use of plication to shorten the annular circumference for treating mitral regurgitation. Starting from the fibrous trigone edge at the base of the large leaflet, a double-armed suture with pledgets is used to perform a semicircular suture along the mitral annulus, reaching the midpoint of the annulus at the base of the posterior leaflet, with each stitch spaced 2–3 mm apart. The suture is then passed through a small pledget, tightened, and tied securely onto the pledget, thereby shortening the annular circumference. If necessary, plication can also be performed on the opposite side of the annulus (Figure 3). Early postoperative mortality was 5.7% in 243 patients. Advanced stage treatment failure, requiring valve replacement, occurred in 16%, with a 3-year postoperative survival rate of 72%.
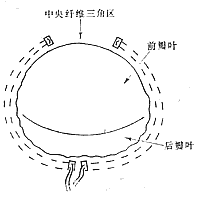
Figure 3 Annular Plication
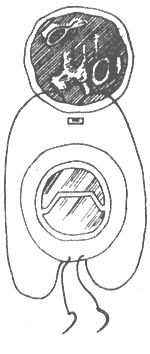
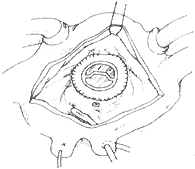
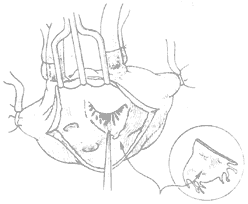
Artificial Annuloplasty Ring: In 1971, Carpentier pioneered the use of an artificial ring designed according to the normal morphology of the mitral valve annulus to treat mitral regurgitation, achieving excellent results. The early artificial rings were made of thick stainless steel wire, later replaced by titanium wire, and coated with synthetic fiber fabric, providing a certain degree of elasticity. When sutured circumferentially to the annulus, it not only shortens the annular circumference but also restores the normal shape of the mitral valve annulus, avoiding valve stenosis and leaflet wrinkling. The left atrium is incised to expose the mitral valve membrane. A right-angle forceps is used to traction the chordae of the anterior leaflet to unfold it. An appropriately sized artificial ring is selected based on the measured area of the anterior leaflet. At the anterolateral and posteromedial commissures, one 2-0 mattress suture is placed through the annulus and the corresponding part of the artificial ring. Then, starting from near the midpoint of the anterior leaflet base annulus, interrupted mattress sutures are used to secure the anterior leaflet annulus to the artificial ring. The stitch intervals between the annulus and the artificial ring are roughly equal. When suturing the posterior leaflet annulus, the stitch intervals on the posterior leaflet annulus should be wider than those on the artificial ring, depending on the degree of annular dilation, to shorten the circumference of the posterior leaflet annulus (Figure 4). After all sutures are placed, the artificial ring is pushed toward the atrioventricular ring. Saline is injected under pressure into the left ventricle to test the closure of the mitral valve. If satisfactory, the sutures are tied one by one. After the artificial ring is placed, if the leaflets coapt well, the coaptation line of the anterior and posterior leaflets will be parallel to the artificial ring at the base of the posterior leaflet.

⑴Measuring the leaflet area
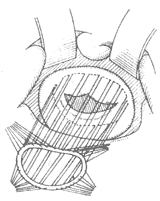
⑵Suturing the artificial annulus to the mitral valve annulus
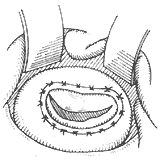
⑶Completion of annuloplasty
Figure 4 Artificial annulus annuloplasty
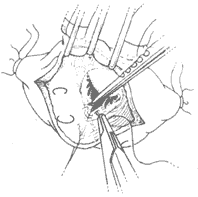
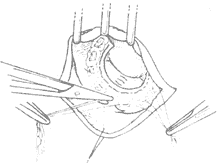
(2) Mitral valve membrane plastic repair In the past 20 years, significant progress has been made in mitral valve membrane plastic repair. Currently, about half of the cases of mitral regurgitation can be improved by plastic repair of the autologous valve membrane to enhance its opening and closing function, thereby avoiding valve replacement, which has a higher incidence of postoperative complications. The operative methods for mitral valve membrane plastic repair depend on the specific conditions of the valve membrane lesions. In some cases, artificial annulus annuloplasty may need to be performed concurrently. Advances in cross-sectional echocardiography have been very helpful in preoperative case selection. Cases of mitral regurgitation with no significant calcification in the valve membrane and subvalvular tissues, and good leaflet mobility, can be considered for plastic repair. Mitral regurgitation can be caused by annular dilation, excessive leaflet mobility leading to the free edge of the leaflet being positioned above the closure line of the valve orifice during left ventricular contraction, or restricted leaflet mobility affecting its opening and closing function. The above conditions may also coexist in the same case. After incising the left atrium to expose the mitral valve membrane, first carefully assess whether the annulus is dilated, whether chordae tendineae and/or papillary muscles are ruptured or excessively long causing excessive leaflet mobility, and whether leaflet mobility is restricted by commissural fusion, leaflet thickening, or chordal fusion. Then, corrective procedures are performed according to the different pathological conditions. Correction of annular dilation mainly involves performing annuloplasty or using an artificial annulus for annuloplasty, sometimes in conjunction with other corrective procedures. Excessive mobility of the posterior leaflet causing prolapse into the left atrium is often due to ruptured or excessively long chordae tendineae. A rectangular section of the affected leaflet and annular tissue can be excised, followed by suturing the annulus and leaflet edges, and then performing annuloplasty with an artificial annulus (Figure 5). For anterior leaflet prolapse caused by excessive mobility, if due to ruptured chordae, the free edge of the prolapsed portion of the anterior leaflet can be fixed with 2–3 sutures to adjacent thicker secondary chordae, or thicker chordae from the posterior leaflet corresponding to the prolapsed portion of the anterior leaflet can be selected to correct the prolapse. A triangular section of the posterior leaflet where the selected chordae are located is excised, the posterior leaflet edges are sutured, and the separated posterior leaflet chordae are fixed to the prolapsed portion of the anterior leaflet with mattress sutures. For anterior leaflet prolapse caused by excessively long chordae, the redundant length of the chordae can be embedded and sutured into a short incision at the apex of the papillary muscle. For mitral regurgitation caused by restricted leaflet mobility, corrective measures include incising fused commissures, excising thickened secondary chordae that restrict leaflet movement, or performing fenestration on thickened primary chordae at the commissural edges. Removing triangular fibrous tissue from thickened chordae can free the leaflets and relieve subvalvular stenosis. Concurrent artificial annulus annuloplasty is required if annular dilation is also present.
⑴Posterior leaflet defect
⑵Ruptured chordae can cause regurgitation; after excising part of the posterior leaflet and annulus, the edges are approximated and sutured
 |  |
(3) Partial resection of the prolapsed posterior leaflet, suturing and shortening the posterior annulus | (4) Correction of leaflet prolapse |
 |  |
| (5) | (6) |
 | 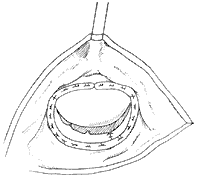 |
(7) Suturing the leaflet cleft | (8) Annuloplasty |
Figure 5 Mitral valve repair
(III) Mitral valve replacement Mitral valve replacement is a commonly used surgical method for treating mitral regurgitation. However, there are still many potential complications after valve replacement. Therefore, when the valve condition permits, repair surgery should be prioritized over replacement. Nevertheless, if the valve is severely damaged, with fibrotic hardening, thickening, contraction, loss of mobility, or calcification of the subvalvular tissue, and the patient's own valve cannot be repaired, valve replacement is necessary.
The clinical application of artificial valves marks a significant advancement in modern cardiothoracic surgery. Over the past 30 years, through continuous research and innovation by medical and engineering professionals, dozens of types of artificial valves have been developed. An ideal artificial valve should have: (1) excellent hemodynamic performance; (2) no thrombus formation; (3) good biocompatibility with human tissue; (4) minimal damage to blood components; (5) ease of implantation; (6) durability, resistance to deformation, damage, or breakage; (7) no disturbance to the patient. Current artificial heart valves do not fully meet all these requirements and require further improvement. Artificial heart valves can be divided into two main categories: mechanical valves made of synthetic materials and biological valves made of physiological tissues. Commonly used mechanical valves include various forms of caged-ball valves, caged-disc valves, tilting-disc valves, and bileaflet-disc valves. Caged-ball and caged-disc valves have poorer hemodynamic performance due to the valve body being centrally located in the blood flow field, requiring blood to pass around the ball or disc, resulting in higher transvalvular pressure gradients, higher thrombosis rates, and greater red blood cell damage. Tilting-disc and bileaflet-disc valves offer lower resistance to blood flow, approximating semi-central or central flow patterns, with better hemodynamic performance, reduced thrombosis rates, and less damage to blood components. In recent years, mechanical valves made of isotropic carbon have further improved wear resistance and physical strength. However, none of the existing mechanical heart valves can completely eliminate the risk of postoperative thromboembolism, necessitating long-term or lifelong anticoagulation therapy after surgery.
During the development of artificial biological valve membranes, various autologous, allogeneic, and xenogeneic tissues, as well as sterilization and preservation methods, have been applied. Clinically, the most commonly used include porcine aortic valve membranes, bovine pericardial valve membranes, and homologous dura mater valve membranes. Artificial biological valve membranes are of the central blood flow type, closely resembling the function of normal artificial valve membranes. They exhibit excellent hemodynamic performance, cause minimal damage to blood components, and have a low incidence of thromboembolism. Postoperatively, lifelong anticoagulation is not required, thereby avoiding bleeding complications caused by excessive anticoagulant medication. They are suitable for patients with bleeding tendencies, women of childbearing age, and cases in remote rural areas where anticoagulation therapy is inconvenient. The major drawback of artificial biological valve membranes is the degenerative changes in biological tissues, leading to calcification, stiffness, rupture, deterioration, and loss of function, necessitating reoperation for replacement. After mitral valve replacement using artificial biological valve membranes, the average annual incidence of valve deterioration in clinical cases is 2%. There is a trend of accelerated deterioration rates five years post-replacement, with even higher rates observed in patients under 15 years of age. Some cases exhibit valve deterioration as early as one and a half years after surgery. In recent years, research has begun on artificial biological valve membranes made from cryopreserved allogeneic fresh aortic valve membranes using liquid nitrogen to maintain cell viability. Ten-year follow-up studies show that the incidence of deterioration in these biological valve membranes is significantly reduced, with markedly improved durability.
Operative Technique of Mitral Valve Replacement: A midline incision is made in the anterior chest, and the sternum is longitudinally split. The pericardium is incised to expose the heart. After systemic heparinization, venous drainage cannulas are inserted into the superior and inferior vena cava via the right atrial appendage and right atrial incision, or a single right atrial drainage cannula is used. An arterial perfusion cannula is inserted into the ascending aorta to establish extracorporeal circulation, lowering the systemic temperature to approximately 25°C. Cold saline is injected into the pericardial cavity to further reduce the local myocardial temperature to around 15°C. The ascending aorta is clamped, and cold cardioplegic solution is injected under pressure at the root of the ascending aorta. A long incision is made in the left atrium along the interatrial groove to expose the left atrium and the mitral valve. If a thrombus is present in the left atrial appendage, it is removed. The mitral valve pathology is examined, and if the valve leaflets are severely damaged and unsuitable for repair, valve replacement is performed. The anterior leaflet is retracted using a mosquito clamp or traction sutures placed at the mid-free edge. An incision is first made at the base of the anterior leaflet, approximately 2–3 mm from the annulus, where the leaflet tissue is usually more pliable and easier to manipulate. The anterior and posterior leaflets are then excised along the annulus, maintaining a distance of 2–3 mm from the annulus using a scalpel or scissors. At the commissures, the chordae tendineae and the tips of the papillary muscles are also excised. After the mitral valve is excised, the annulus size is measured using an annulus sizer. Based on the patient’s age, sex, social and economic status, and annulus size, an appropriate type and size of prosthetic valve are selected. Twelve to sixteen mattress sutures of size 0 polyester sutures, each armed with a non-traumatic needle at both ends and reinforced with small polyester pledgets, are placed from the atrial side through the annulus to the ventricular side. When placing these mattress sutures, care should be taken to distribute them evenly around the entire circumference of the annulus. Each pair of mattress sutures is sequentially clamped with mosquito clamps to avoid confusion. After all the annular sutures are placed, each pair of mattress sutures is precisely passed through the corresponding points on the sewing ring of the prosthetic valve. The prosthetic valve is then lowered into the annular position while all mattress sutures are tightened. After ensuring the sewing ring is snug against the annulus, the sutures are tied individually, with 5–6 knots for each mattress suture. The excess suture material should not be left too long to avoid interference with the valve orifice. An alternative suturing technique involves initially securing the annulus to the sewing ring of the prosthetic valve with a single pledgeted suture. The prosthetic valve is then positioned in the annulus, and continuous suturing is performed from both ends of the suture to attach the annulus to the sewing ring (Figure 6). To ensure unobstructed blood flow after the prosthetic valve is placed in the left ventricular cavity, the optimal orientation of the prosthetic valve should be selected based on its design. For tilting disc valves, the larger opening should face the posterior left ventricular wall; for porcine aortic valves, the right coronary leaflet should be positioned toward the ventricular septum; and for bovine pericardial valves, the struts should avoid the left ventricular outflow tract. After the prosthetic valve is sutured in place, a fine catheter is inserted through the prosthetic valve orifice or via a small incision at the left ventricular apex to remove residual air from the left heart chambers. The left atrial incision is closed, the aortic clamp is released, and a venting needle is inserted at the root of the ascending aorta. Once the heart resumes strong contractions and the body temperature is raised to approximately 35°C, extracorporeal circulation is discontinued. The venous and arterial cannulas are removed, and the pericardial edges are sutured, leaving a small opening for postoperative drainage. A drainage tube is placed in the pericardial cavity and another in the anterior mediastinum. The sternum is reapproximated and secured with wire sutures. The surgical incision is closed in layers.
|
|
|
⑴ | ⑵ |
|
|
|
| (3) | (4) |
Figure 6 Mitral valve membrane replacement
[Treatment efficacy] The surgical mortality rate of mitral valve membrane plasty is approximately 4-5%, with the most common causes of death being left ventricular failure and arrhythmia. 10% of patients require reoperation due to residual mitral valve insufficiency. The mortality rate in advanced stages is 7%, primarily due to recurrence of insufficiency leading to reoperation. Postoperatively, 76% of patients recover to NYHA Class I heart function, 11% to Class II, totaling 87%, with an annual thromboembolism incidence of 0.6%.
The surgical mortality rate of mitral valve membrane replacement is about 8-10%. In 75% of cases, heart function improves from preoperative NYHA Class III-IV to Class I-II. Clinical symptoms are significantly alleviated, exercise capacity increases, and cardiac shadow gradually reduces, potentially returning to normal size. The 5-year, 10-year, and 15-year survival rates postoperatively decrease to 80%, 60%, and 45%, respectively. Adverse factors affecting efficacy include prolonged disease course, preoperative NYHA Class III-IV heart function, left ventricular dysfunction, significant cardiac enlargement, pulmonary arterial hypertension, atrial fibrillation, advanced age, concurrent coronary atherosclerotic heart disease, and reoperation. Complications after valve membrane replacement are related to the type of prosthetic mitral valve used and may include chronic hemolytic anemia, perivalvular fistula bleeding, thromboembolism, prosthetic valve endocarditis, valve damage or failure, and hemorrhage in the cranium or other sites due to excessive anticoagulant medication.