| disease | Vasomotor Rhinitis |
| alias | Vasomotor Rhinitis |
Vasomotor rhinitis is a hyperreactive nasal disease caused by an imbalance in the neuroendocrine regulation of nasal mucosal blood vessels and glandular function. The pathological mechanisms of this condition are intricate, with many aspects still poorly understood, which poses certain challenges for clinical diagnosis and effective treatment. There is no significant gender difference in its occurrence, and vasomotor rhinitis rarely affects children.
bubble_chart Pathogenesis
The nasal mucosa contains a large number of glands, a rich vascular bed, and diverse sources of innervation, forming a delicate, sensitive, and active terminal organ that performs various physiological functions as the gateway of the respiratory tract. It relies on neurovascular and neuroendocrine activities to maintain the balance between the nasal cavity and the internal and external environments. This balance superficially depends on two pathways originating from the hypothalamus: one involves the pituitary gland regulating nasal mucosal functions through the endocrine chain via humoral regulation, while the other directly involves neural regulation through the autonomic nervous system. If functional changes occur in either of these pathways, it can lead to dysfunction of nasal mucosal blood vessels and glands, as well as heightened reactivity, which constitutes the primary pathophysiological basis for the onset of vasomotor rhinitis.
(1) Autonomic Nervous System Dysfunction
Under normal conditions, when the sympathetic nerves are stimulated, their terminals release norepinephrine and neuropeptide Y, maintaining vascular tension in the nasal mucosa through corresponding receptors on the vascular walls. When the parasympathetic nerves are stimulated, their terminals release acetylcholine, causing vasodilation and glandular secretion via M receptors on the vascular walls and glands. Recent studies have also identified vasoactive intestinal peptide (VIP)-immunoreactive fibers in the parasympathetic nerves of the nasal mucosa. Stimulation of the vidian nerve (primarily containing parasympathetic fibers) releases VIP from these fibers, which induces vasodilation that cannot be blocked by atropine. Uddman (1987) proposed that glandular secretion caused by parasympathetic stimulation is mediated by acetylcholine, while vasodilation is attributed to a non-cholinergic vasodilator—VIP.
Repeated sympathetic stimulation (e.g., overwork, dysphoria, anxiety, or stress) can deplete excessive neurotransmitter synthesis enzymes and pre-synthesized neurotransmitters stored in nerve terminals, leading to a corresponding reduction in the number of α1 and β receptors, thereby lowering sympathetic tone. Certain antihypertensive drugs, non-selective β-blockers, and antidepressants act as sympathetic blockers, and their repeated use can also reduce sympathetic tone. When sympathetic tone is diminished, parasympathetic excitability increases, leading to vasodilation and excessive glandular secretion, resulting in the clinical symptoms of vasomotor rhinitis. As early as 1943, Fowler observed that cervical sympathectomy could induce vasomotor rhinitis in patients. Removal of the superior cervical sympathetic ganglion in animals caused nasal mucosal vasodilation, submucosal edema, glandular hyperplasia, and increased cholinesterase activity around the glands—changes identical to the histopathological findings in vasomotor rhinitis patients.(2) Endocrine Dysfunction
Endocrine imbalances can also alter nasal mucosal reactivity. Hypothyroidism can reduce sympathetic tone, often manifesting as a primary symptom of nasal congestion. Changes in estrogen levels can also cause nasal symptoms. Numerous clinical reports indicate that some female patients experience significant nasal symptoms—such as nasal congestion, frequent sneezing, and clear rhinorrhea—during the premenstrual period or pregnancy. Animal studies have confirmed that elevated estradiol levels markedly enhance nasal mucosal reactivity, leading to epithelial thickening, tissue edema, small vessel dilation, and glandular hyperplasia. The mechanism of estradiol’s action remains unclear, but it has been observed that increased estradiol levels elevate cholinergic M-receptors and reduce α1-adrenergic receptors in nasal mucosal tissue. Estradiol also enhances non-immunological histamine release from mast cells.
Non-immunological Release of Histamine There are various factors that can cause the non-immunological release of mediators such as histamine, including chemical factors (anesthetics, salicylates), physical factors (sudden changes in temperature, humidity, climate, dust), and neurological factors (emotional changes). The exact mechanism of non-immunological histamine release remains unclear, but regardless of the precise mechanism, it is regulated by intracellular cAMP levels. As long as intracellular cAMP levels decrease, mast cells can release mediators.
bubble_chart Pathological Changes
Histological changes in the nasal mucosa show an increase in goblet cells, vascular dilation, glandular hyperplasia, and tissue edema. Unlike allergic rhinitis, there is minimal granulocyte infiltration in the tissue, and the endothelial cell junctions of small blood vessels remain intact.
bubble_chart Clinical Manifestations
According to the disease causes, Goldman (1987) classified vasomotor rhinitis into three clinical types, which holds certain significance for diagnosis and treatment.
(1) Physical Reaction Type
Some patients often exhibit specific nasal reactions to certain physical stimuli. For example, exposure to cold air, sudden temperature changes, humidity, etc., can trigger paroxysmal sneezing accompanied by excessive watery nasal discharge. Patients can usually clearly identify the inducing factors. This type may also belong to hyperreactive rhinitis.
(2) Psychogenic Reaction Type
Repeated psychological stimuli such as tension, fear, resentment, or depression can induce nasal reactions in patients. The contradiction between the changes in modern neurotic lifestyles and the accelerated pace of life, compared to traditional concepts, has led to an increase in the number of such patients.
For this type, no identifiable triggering factors can be found, and it accounts for the majority of vasomotor rhinitis cases. Patients often experience watery nasal discharge, nasal mucosal edema, and sometimes mucosal polyps or nasal polyps. Endocrine dysfunction may be one of the contributing factors.
Based on clinical characteristics, it can be further divided into two subtypes:
1. Stuffy Nose Type
The primary symptom of this type is nasal congestion, mostly intermittent. Some patients experience severe nasal congestion upon waking in the morning, which diminishes or disappears during the day. Others may have worsening symptoms at night, often accompanied by alternating nasal congestion that changes with body position. If the nasal mucosa develops polypoid changes or nasal polyps, varying degrees of persistent nasal congestion may occur. Sneezing may occur occasionally but is usually mild, with temporary relief after sneezing. Patients are often highly sensitive to changes in climate and environmental temperature.
2. Rhinorrhea Type
The main symptom is increased watery nasal discharge, often accompanied by paroxysmal sneezing. Episodes typically last for several consecutive days, requiring multiple handkerchief changes or the use of large amounts of tissue paper. Nasal itching is common, but symptoms such as conjunctival involvement or eye itching are rare. Symptoms may spontaneously lessen or disappear after several days or weeks, only to recur under certain triggers after a certain interval. This type is more common in women aged 20–40, often with unstable psychological states.
Other symptoms include decreased sense of smell, dizziness, etc., caused by persistent mucosal swelling, congestion, and edema.
Rhinoscopy Findings {|111|} The color of the nasal mucosa shows no consistent changes. It may appear dark red due to congestion, light blue due to dilated capacitance vessels, or pale due to mucosal edema. In some cases, one side of the nasal mucosa may be congested and dark red, while the other side appears pale and edematous. The turbinates in patients with hypertrophy generally respond well to ephedrine-induced contraction, but those with long disease duration or repeated use of nasal decongestant drops may show poor contraction response. Long-term cases may exhibit mucosal edema and polypoid changes. Posterior rhinoscopy may reveal enlarged and edematous posterior ends of the inferior turbinates.
Almost everyone experiences occasional nasal symptoms, making it sometimes difficult to distinguish between a normal nose and a diseased one. However, by carefully inquiring about medical history, conducting thorough examinations, and analyzing triggering factors in detail, a diagnosis of vasomotor rhinitis can be made if nasal symptoms accumulate for more than one hour daily and persist for over a month, provided the following conditions are excluded.
1. Allergic Rhinitis: Positive allergen skin test, with eosinophils and basophils present in nasal secretions. Seasonal rhinitis occurs in a seasonal pattern.
2. Infectious Rhinitis: Can be acute or chronic. Nasal secretions are typically mucous or mucopurulent, with predominantly neutrophils in the secretions.
3. Non-Allergic Rhinitis with Eosinophilia Syndrome (NARES): Large numbers of eosinophils are found in nasal secretions, but there is no other evidence of allergic reactions.
4. Aspirin-Exacerbated Respiratory Disease (AERD): Although nasal secretions may contain many eosinophils, patients have a history of hypersensitivity to salicylates or other antipyretic analgesics and a history of asthma, along with nasal polyps.
5. Hyperreflexive Rhinitis: Caused by excessive reflex of sensory nerve axons in the nose, characterized by sudden sneezing as the main symptom, with abrupt onset and rapid resolution.
bubble_chart Treatment Measures
Since the disease has multiple inducing factors and a complex pathogenesis, comprehensive measures should be taken during treatment.
(1) Avoid or eliminate inducing factors
Improve working conditions and environment, maintain a balanced lifestyle, stabilize emotions, and avoid excessive fatigue and stress. Necessary psychological therapy or suggestive language for patients can sometimes yield significant results. For cases caused by endocrine factors, consultation with an endocrinologist should be considered for collaborative treatment.
(2) Drug therapy
Medications should be selected based on the progression of the condition.
1. Nasal decongestants: These can be chosen for patients whose main symptom is stuffy nose. However, the risk of drug-induced rhinitis should be noted during use. Intermittent or alternating administration is recommended. Adenosine triphosphate sodium (ATP), 40mg each time, three times daily, has shown significant efficacy in relieving stuffy nose. Recent studies suggest that ATP may function as another sympathomimetic drug.
2. Antihistamines: Many non-immune factors can trigger mast cells to release histamine, so antihistamines Yaodui remain effective in many cases. For patients with prominent nasal itching and sneezing, antihistamines can be the first choice.
3. Anticholinergic drugs: Suitable for patients whose main symptom is rhinorrhea. Ipratropium bromide aerosol, 80μg per nostril, four times daily, can effectively control rhinorrhea.
4. Adrenocorticosteroids: Corticosteroids exert non-specific anti-inflammatory effects at various intracellular and extracellular levels, making them highly effective for cases of vasomotor rhinitis with pronounced sneezing, watery nasal discharge, and significant nasal mucosal edema.
(3) Surgical treatment
Surgical intervention may be considered under the following circumstances: ① Symptoms persist and worsen after conservative treatment for over a year; ② Anatomical deformities in the nasal structure significantly impair ventilation or sinus drainage; ③ Irreversible pathological changes such as proliferative nasal mucosal alterations or large polyps.
1. Correction of anatomical deformities: Nasal structural deformities that exacerbate vasomotor rhinitis symptoms mainly include deviated nasal septum, which in severe cases may contact or press against the turbinates. Such prolonged stimulation not only aggravates local inflammatory reactions but often leads to headache. Narrow nasal passages, often caused by collapse of the lateral nasal cartilage, are another common anatomical deformity primarily causing stuffy nose. Nasal symptoms arising from such deformities are sometimes termed structural rhinitis. Early correction of these deformities can significantly alleviate symptoms or even cure the condition.
2. Resection of irreversible pathological tissue: Hyperplastic and hypertrophic turbinates that cause significant stuffy nose, as well as nasal polyps formed by long-term mucosal edema, should be promptly removed.
3. Reduction of nasal nerve excitability: Severing parasympathetic nerve fibers innervating the nasal cavity to reduce excitability. Such procedures include:
(1) Greater superficial petrosal neurectomy: First proposed by Ziegelman (1934) for treating vasomotor rhinitis. In China, Fan Zhong (1987) reported significant short- and long-term efficacy in 11 cases treated with this method. However, the procedure requires craniotomy, which is generally less acceptable to patients.
The greater petrosal nerve is purely parasympathetic fibers originating from the lacrimal nucleus in the pons. After entering the internal auditory canal, it is called the intermediate nerve, descends to the geniculate ganglion of the facial nerve, and then proceeds forward within the petrous bone canal after emerging from the ganglion. Exiting the hiatus of the facial nerve canal, it runs externally to the dura mater in the groove for the greater petrosal nerve on the anterior surface of the petrous bone. It passes below the trigeminal ganglion or the mandibular nerve to reach the foramen lacerum, where it merges with the deep petrosal nerve (derived from the sympathetic plexus around the internal carotid artery) to form the vidian nerve. According to Lin Yuanwen et al. (1985), a study of 30 adult cadavers (60 sides) showed that the total length of the greater petrosal nerve is 15.38±1.91 mm. The proximal segment (bony canal portion) measures 3.64±1.4 mm, while the distal segment (groove portion) is 11.73±2.69 mm. The entire nerve is relatively thin, with a transverse diameter of approximately 0.42±0.09 mm, accompanied by small blood vessels. The blood supply mostly comes from the posterior branch of the middle meningeal artery. The depth of the groove segment varies; in 7 cases (11.6%), the nerve trunk was completely hidden within the groove, with the edges of the groove forming a very narrow bony fissure over the superficial aspect of the nerve trunk, making it somewhat difficult to sever the nerve within the groove.
Lateral to the greater superficial petrosal nerve is the lesser superficial petrosal nerve, which runs almost parallel to it. The average transverse diameter is 0.36±0.09 mm. Most of the nerve runs within the bony canal, while a few traverse a groove. Care must be taken during surgery to avoid mistaking it for the greater superficial petrosal nerve.
The hiatus of the facial nerve canal is approximately 1 cm from the foramen spinosum anteriorly and the arcuate eminence posteriorly, all lying in a straight line. At the hiatus of the facial nerve canal, there is a silvery-white dense connective tissue membrane adherent to the dura mater, serving as an important anatomical landmark during surgery.
The surgical approach is the same as the Spiller-Frazier trigeminal sensory root section, and the middle cranial fossa approach via the temporal bone may be used. The surgical field is relatively large. The patient is placed in a semi-sitting position to reduce intracranial pressure and minimize bleeding in the operative area. General anesthesia with endotracheal ether or local procaine reinforcement is administered. A 7 cm longitudinal skin incision is made 3 cm anterior to the tragus, extending from above the zygomatic arch posteriorly. The temporalis muscle and periosteum are separated, and a mastoid retractor is used to retract them laterally. An electric drill and rongeur are used to create a circular craniotomy window 4–5 cm in diameter in the squamous part of the temporal bone, extending as low as possible toward the cranial base. The dura mater of the cranial base is dissected from lateral to medial, first revealing the arcuate eminence, followed by locating the foramen spinosum along the pulsating middle meningeal artery. The greater superficial petrosal nerve lies between the arcuate eminence and the foramen spinosum, running roughly parallel to the long axis of the petrous bone, approximately 1 cm from each landmark. Its posterolateral segment enters the petrous bone to reach the geniculate ganglion, while its anteromedial segment passes beneath the trigeminal ganglion into the foramen lacerum. At this point, gently dissecting slightly anteromedial to the arcuate eminence with a dissector reveals reddish nerve fibers. The greater superficial petrosal nerve is often adherent to the dura mater by fibrous tissue, so dissection must be performed carefully to avoid forceful tearing, which could injure the geniculate ganglion and cause facial deviation. Once the greater superficial petrosal nerve is identified, it is best to section or cauterize it in situ within its groove rather than lifting or retracting it to prevent facial deviation. Gardner and Nosik (1951) recommended excising a 2–4 mm segment of the nerve to prevent regeneration and symptom recurrence. In cases with an exceptionally deep groove, a microelectrode may be inserted into the fissure of the groove to cauterize and destroy the nerve. Care must be taken to avoid proximity to the hiatus of the facial nerve canal to prevent injury to the facial nerve. Any bleeding can be controlled with cautery, followed by layered closure of the incision. Postoperative antibiotics are administered to prevent infection.
(2) Vidian Neurectomy The vidian nerve contains parasympathetic fibers entering the nasal cavity. Malcomson (1959) first proposed transnasal septal neurectomy of the vidian nerve. Subsequently, various surgical techniques have been reported, all claiming good efficacy, though long-term outcomes vary. This procedure can control sneezing and watery rhinorrhea but is less effective for nasal congestion. Postoperative dry eye discomfort is a common complication.
The vidian nerve consists of preganglionic parasympathetic fibers from the greater superficial petrosal nerve and postganglionic sympathetic fibers from the deep petrosal nerve. These two types of fibers converge at the foramen lacerum, then travel forward within the vidian canal, where they are collectively termed the vidian nerve. This nerve exits the vidian canal anteriorly into the medial pterygopalatine fossa, where it joins the sphenopalatine ganglion superiorly and deeply. From here, postganglionic parasympathetic and sympathetic fibers are distributed via the zygomatic branch of the maxillary nerve, communicating with the lacrimal nerve to supply the lacrimal gland.
The external opening of the vidian canal is funnel-shaped, located inferolateral to the sphenoid body, at the apex of the medial pterygoid plate, inferomedial to the foramen rotundum, and inferolateral to the natural ostium of the sphenoid sinus. A bony ridge separates the foramen rotundum from the external opening of the vidian canal. The author observed that inserting a rounded probe through the anterior naris along the middle meatus, directed posterolaterally about 1 cm above the choana, allows palpation of the funnel-shaped depression marking the external opening of the vidian canal, serving as a surgical landmark.
The relationship between the sphenopalatine foramen and the posterior end of the middle turbinate: Among 100 cases, the sphenopalatine foramen was located posterior to the middle turbinate in 95% and superior to it in 5%.
① Transmaxillary Approach This can be divided into the following four methods:
Golding-Wood method: Make an incision according to the Caldwell-Luc maxillary sinus radical surgery approach, create a bone window on the anterior wall, and incise the posterior wall mucosa to form a square mucosal flap, which is then turned downward. Remove the posterior wall bone, taking care not to injure the underlying periosteum. After achieving adequate hemostasis, use a blunt instrument to separate the periosteum and enter the pterygopalatine fossa. Dissect the adipose tissue to locate the internal maxillary artery and occlude it with a silver clip. Carefully dissect deeper, avoiding injury to small veins to prevent bleeding. Expose the anterior surface of the sphenoid bone and locate the foramen rotundum, through which the maxillary nerve passes. Touching this area will cause pain in the patient. From the foramen rotundum, search downward within 1 cm to find a funnel-shaped depression, which is the opening of the vidian canal. Use a small knife to sever the tissue emerging from the vidian canal opening, and cauterize the cut end with an electric knife or seal the vidian canal opening with bone wax. The subsequent steps are the same as those in the Caldwell-Luc procedure.
Nomura's Method: Unlike the Golding-Wood method, after opening the maxillary sinus, the posterior medial angle of the mucous membrane is cut into a flap and lifted upward to expose the bone wall in that area. The pterygoid canal orifice is located at the junction of the posterior medial wall of the maxillary sinus, specifically behind the midpoint between the top and bottom. First, the bony part of the posterior wall at the junction is removed to create a window. Then, the opening is expanded inward, and part of the medial wall of the maxillary sinus is removed. A bone elevator is inserted between the mucous membrane of the lateral nasal wall and the mucous membrane of the posteromedial angle of the maxillary sinus. Since the distance between the posterior wall of the maxillary sinus and the anterior surface of the sphenoid bone increases toward the cranial end and decreases toward the caudal end—even fusing completely—the elevator should be guided from the caudal end along the anterior surface of the sphenoid bone toward the cranial end to locate the pterygoid canal orifice. Once found, the tissue at the orifice is first cauterized with an electric knife, and then the emerging tissue from the orifice—including the pterygoid canal nerve, pterygoid canal artery, and connective tissue—is severed. Bone wax can be packed into the orifice. The mucous membrane flap is repositioned, and an antrostomy is performed as in the Caldwell-Luc operation, followed by suturing the incision. This method eliminates the need to dissect structures in the pterygopalatine fossa, saving time.
Legent's Method: First, the ethmoid sinus is opened via the maxillary sinus (de Lima operation), and the ethmoid-maxillary sinus cell is identified. The opening is expanded outward to the medial orbital wall and downward to the lower ridge of the cell. Here, along the medial wall of the maxillary sinus, the pterygoid canal orifice is located precisely at the medial segment of the lower ridge of the ethmoid-maxillary sinus cell and the plane of the medial maxillary sinus wall. The pterygoid canal nerve is severed using the aforementioned method. This approach is particularly suitable for treating recurrent nasal polyps.
Bu Guoxuan's Method: First, the ethmoid sinus is opened via the maxillary sinus. A round-headed probe is inserted along the middle nasal meatus from the nostril. Approximately 1 cm above and lateral to the posterior nasal aperture, a funnel-shaped depression—the pterygoid canal orifice—is palpated. A small vertical bony crest lies superolateral to it. Crossing this crest and probing laterally will elicit severe pain (due to contact with the maxillary nerve in the foramen rotundum), which serves as a reference for locating the pterygoid canal orifice. The depth from the pterygoid canal orifice to the anterior nostril is 6–7 cm. Once the probe is inserted into the orifice, it can be visualized through the window in the anterior maxillary sinus wall, providing a reliable landmark for locating the orifice. The ethmoid air cells and the bony ridge between the ethmoid and maxillary sinuses are cleared to fully expose the orifice. A small knife is used to sever the nerve, artery, and fibrous tissue emerging from the orifice. Pure carbolic acid is applied to cauterize the residual stump, avoiding electrocautery to prevent ocular complications from electrical stimulation. Carbolic acid cauterization also stops bleeding from the pterygoid canal artery. To prevent nerve regeneration, bone wax can be used to seal the pterygoid canal orifice. This method is suitable for treating recurrent nasal polyps. For research purposes, a small segment of the pterygoid canal nerve can be excised during surgery for pathological examination (Figure 1).
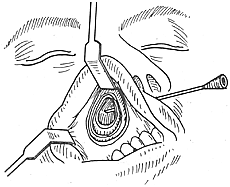
Figure 1: Transmaxillary Pterygoid Canal Neurectomy
Laser Neurectomy: Williams (1983) reported using an 8W CO2 laser beam to sever the pterygoid canal nerve at the medial posterior maxillary sinus wall during surgery, with good results in 12 cases and minimal postoperative reactions.
② Transnasal Approach: Further divided into the following three methods:
Malcomson's Method: Also known as the transseptal approach. Introduced in 1959 and later adopted by Minnis (1971). The method involves performing a submucous resection of the nasal septum, followed by posterior dissection of the mucoperiosteum until reaching the anterior wall of the sphenoid sinus. Further lateral dissection extends to the root of the pterygoid process, approximately 1 cm above the posterior bony margin of the posterior nasal aperture, where the funnel-shaped depression of the pterygoid canal orifice can be palpated. Under good illumination, the pterygoid canal orifice may sometimes be visualized. An electrocautery needle is then inserted into the orifice to destroy the pterygoid canal nerve. Advantages of this method include minimal tissue injury, relative simplicity, the ability to perform bilateral surgery in one session, and low infection risk. However, it carries the drawbacks of potential directional deviation and blind manipulation.
The Patel method, also known as the direct middle meatal approach. Under local anesthesia, the bilateral inferior turbinates are fractured laterally, and the middle turbinate is fractured medially to widen the surgical space. A Killian long nasal speculum is inserted to visualize the superolateral wall of the posterior nasal aperture. Using the attachment site of the middle turbinate as a landmark (to be decocted later), the mucoperiosteum is incised and dissected circumferentially to locate the sphenopalatine foramen and the vidian canal. The semicircular sphenopalatine foramen is first identified, from which fibrous cord-like structures emerge and connect to the nasal mucosa. A probe is inserted into the sphenopalatine foramen for approximately 0.5 cm; if a fixed sensation is felt, it indicates reaching the vidian canal. A small sickle-shaped knife is then introduced into the canal to sever the vidian nerve and its accompanying vidian artery. If pulsatile bleeding occurs, it can be controlled by applying an adrenaline-soaked gauze strip for several minutes. To prevent nerve regeneration, the nerve stump may be cauterized with an electrode (the author recommends using pure carbolic acid for cauterization), and the vidian canal is sealed with bone wax. Finally, the nasal cavity is packed with Vaseline gauze. The advantage of this method is the shortest operative time. The drawback is that it is not suitable for treating recurrent nasal polyps. During the procedure, the probe may accidentally enter the natural ostium of the sphenoid sinus or the ethmoid air cells, so careful observation is necessary to avoid this. The use of nasal endoscopy can further facilitate the surgery.
Kamel's Method, also known as endoscopic vidian neurectomy via the nasal approach. The patient is placed in a supine position, and the nasal cavity is topically anesthetized with 4% cocaine or 1% tetracaine plus 0.1% adrenaline. The key areas for anesthesia are the middle turbinate, middle meatus, and olfactory cleft. The mucosa of the middle turbinate is infiltrated with 1% lidocaine. Using 0° and 30° nasal endoscopes, the posterior end of the middle turbinate is first fractured laterally to expose the posterior part of the superior meatus. If the posterior end of the middle turbinate is too large, it should be excised. Between the posterior ends of the superior and middle turbinates, the sphenopalatine foramen is identified with a curved probe. A sickle knife is used to make a longitudinal incision through the cartilaginous membrane at the posterior edge of the sphenopalatine foramen, followed by anterior dissection to confirm the posterior edge of the foramen. Careful subperiosteal dissection is then performed transversely and posteriorly until the vidian canal opening is reached, exposing the vidian nerve. Under direct endoscopic visualization, the vidian nerve is severed. If the sphenopalatine foramen is narrow during the procedure, it can be enlarged with a curette, and any bleeding can be controlled with electrocautery. The advantage of this method is that bilateral surgery can be performed simultaneously.
③ Transpalatal Approach Chandra (1969) and Mostafa (1973) performed this procedure under general anesthesia with endotracheal intubation. The pharynx is first packed, and a mouth gag is inserted. A curved incision is made 2 cm anterior to the posterior edge of the hard palate, extending to the last molar on both sides, avoiding the greater palatine artery. The incision is deepened to the bone, and the mucoperiosteum is elevated to expose the palatal aponeurosis. The soft palate is incised at the posterior edge of the hard palate, and approximately 5 mm of the posterior edge of the hard palate is removed to expose the mucosa of the medial pterygoid plate in the posterior lateral wall of the nasopharynx. Using the eustachian tube orifice and torus as landmarks, the procedure is performed under 6x surgical microscopy. The mucosa is injected with 0.1% adrenaline, and an "L"-shaped incision is made. The long limb of the incision is above the torus, extending from posterior to anterior, while the short limb is between the posterior and lateral walls of the nasopharynx, extending from superior to inferior. The mucoperiosteum is elevated to expose the medial pterygoid plate up to its superior edge and its junction with the basiocciput. The foramen lacerum and the internal carotid artery running through it are located posterolaterally to this junction and must not be injured. The vidian canal is located 2–3 mm within the cancellous bone at the superior edge of the medial pterygoid plate and appears as an ivory-like bony canal. The canal is drilled open, the nerve is identified, hooked, and severed, and the central stump is cauterized. The mucosa is then repositioned, and the palatal incision is sutured.
④ Non-Surgical Vidian Neurectomy
Cryotherapy of the Vidian Nerve First introduced by Poch vinals and Poch Brato in 1977. Yin Juzhong (1983) used a BYD-1 cryotherapy device with a modified maxillary sinus puncture needle as the cryoprobe (80 mm long, 1 mm inner diameter, insulated with a silicone sleeve). Following the aforementioned method, the cryoprobe is inserted into the vidian canal via the anterior nostril and cooled to -196°C for 20–30 seconds, forming an ice ball 1.5–2.0 cm in diameter at the canal opening. The probe is rapidly rewarmed and removed, achieving the goal of severing the vidian nerve. This method also destroys the sphenopalatine ganglion. Postoperative reactive headache and eye pain may occur but typically resolve within 1–2 days.
Electrocautery of the Vidian Nerve First performed by Portmann (1982) and Kirtane (1984). Wang Zhongzhi et al. (1987) performed high-frequency electrocautery via the sphenopalatine foramen to the vidian canal under topical nasal anesthesia, achieving an 80% efficacy rate in 408 cases.
Intracanal Injection Therapy These drugs have neurolytic effects, such as 95% alcohol and compound quinine. Song Changxiang and Li Desheng et al. (1984) successfully treated a large number of patients with this method. The authors noted that the superior wall of the vidian canal may have natural bony defects, and the injected volume must be limited to 0.5 ml or less to avoid meningeal irritation.
(3) Anterior Ethmoidal Neurectomy The anterior ethmoidal nerve is the terminal branch of the ophthalmic division of the trigeminal nerve and contains parasympathetic fibers from the ciliary ganglion, distributed to the anterior nasal cavity. The anterior nasal cavity is also a high-density area for nasal serous glands. Based on this, Bu Guoxuan (1989) proposed that simultaneous transection of the anterior ethmoidal nerve and vidian nerve could better control symptoms of vasomotor rhinitis.
The first branch of the trigeminal nerve is the ophthalmic nerve, which arises from the trigeminal ganglion and enters the cavernous sinus before dividing into three branches: the frontal nerve, the lacrimal nerve, and the nasociliary nerve. The nasociliary nerve travels forward through the superior orbital fissure into the orbit, giving off the anterior ethmoidal nerve and the posterior ethmoidal nerve. The parasympathetic fibers originating from the oculomotor nucleus (Edinger-Westphal nucleus) synapse in the ciliary ganglion behind the eyeball within the orbit. These fibers then merge with the anterior ethmoidal nerve, forming a mixed nerve that contains both sensory and parasympathetic fibers. In contrast, the posterior ethmoidal nerve consists solely of sensory fibers without any parasympathetic component.
The anterior ethmoidal nerve runs anteromedially within the orbit, between the superior oblique muscle and the medial rectus muscle, forming a bundle with the anterior ethmoidal artery and vein. It passes through the anterior ethmoidal foramen into the anterior ethmoidal air cells, then traverses horizontally along the roof of the ethmoid sinus into the anterior cranial fossa, located outside the dura mater. It proceeds to the lateral side of the anterior part of the crista galli, passes through a small fissure downward into the nasal cavity. In the anterior-superior part of the nasal cavity, it divides into medial and lateral branches. The former supplies the mucosa of the nasal cavity and frontal sinus, while the latter supplies the skin of the nasal root and dorsum. The medial nasal branch of the anterior ethmoidal nerve further divides into a branch supplying the nasal septum and another supplying the lateral wall of the nasal cavity. The parasympathetic fibers of the anterior ethmoidal nerve within the nasal septum may sometimes enter the perpendicular plate of the ethmoid bone before emerging again to supply the numerous serous glands in the anterosuperior part of the nasal septum. A cross-section of the intraorbital segment of the anterior ethmoidal nerve reveals it is composed of four nerve fiber bundles, which appear brownish-yellow with El-Badawi acetylcholinesterase staining, highlighting the parasympathetic fibers.
① Intraorbital Approach: Under local anesthesia, a curved incision is made along the medial orbital margin, and the medial canthal ligament is severed. Dissection is carried out subperiosteally along the medial orbital wall to a depth of approximately 2 cm from the orbital rim, where the anterior ethmoidal foramen is located. The anterior ethmoidal nerve and artery are seen enclosed within a fibrous bundle. The nerve is then isolated and transected, and the wound is closed in two layers. This method is simple and feasible but may leave postoperative scarring.
② Intranasal Cauterization: Surface anesthesia is applied to the upper part of the nasal septum from front to back. The septal branch of the anterior ethmoidal nerve (located in the anterosuperior part of the nasal septum, near the nasal dorsum, at the level of the infraorbital rim) is severed using electrocautery or electrocoagulation. Only one side is treated at a time. Postoperatively, sneezing and watery nasal discharge may occur but typically resolve within hours. If symptoms recur, the same procedure can be performed on the contralateral side. The long-term efficacy of this method is approximately 86.6%. Inaccurate cauterization may affect the outcome.
③ Ethmoidal Sinus Approach: With the assistance of nasal endoscopy, an intranasal ethmoidectomy is performed to remove the anterior ethmoidal air cells. Under endoscopic visualization, the anterior ethmoidal nerve and artery are seen running transversely along the roof of the ethmoid sinus. The ethmoid sinus roof is thin and bluish, allowing for transection with an electrocautery device. Care must be taken to avoid injury to the roof to prevent damage to the dura mater. This method is suitable for patients who also suffer from nasal polyps.






