| disease | Congenital Tibia Pseudoarthrosis |
Congenital tibia pseudoarthrosis is a condition where developmental abnormalities in the middle to lower third of the tibia lead to deformity and a specific type of nonunion, ultimately resulting in the formation of a localized pseudarthrosis. Since Hatzoecher first described this disease in 1709, it has been over 200 years.
bubble_chart Etiology
There are many theories regarding the causes of deformity. The intrauterine compression theory suggests that the fetus in the uterus has the foot in extreme dorsiflexion, pressing on the lower one-third of the tibia, severely affecting its blood supply. Some believe it is due to intrauterine trauma, resulting in a fracture at that site and leading to deformity. However, more people consider it a disease caused by a systemic metabolic disorder; almost all patients exhibit skin pigmentation spots, and the local area often coexists with the presence of neurofibromas. Some scholars also propose that congenital tibial pseudoarthrosis and fibrous dysplasia of bone may belong to the same disease etiology, merely manifesting different clinical presentations. Aegerter, on the other hand, suggests that fibrous dysplasia of bone, neurofibromatosis, and congenital tibial pseudoarthrosis all arise from neural variations that disrupt tissue growth and maturation. The local bone membrane at the pseudoarthrosis site is often very thick, forming a dense fibrous tissue sleeve. This hamartomatous proliferation of soft tissue interferes with bone growth and the formation of normal callus, while the thickened fibrous tissue tightly adhering to the bone cortex restricts blood supply, leading to bone atrophy. Similar conditions can be observed in the clavicle, ribs, femur, and humerus, though they are extremely rare.
bubble_chart Clinical Manifestations
The typical congenital tibia pseudoarthrosis deformity manifests as anterior bowing or a pseudoarthrosis appearance in the middle third of the child's lower leg, with limb shortening.
Based on tibial morphology, it is generally classified into three types clinically.
(1) Bowing Type: After birth, the lower segment of the tibia bows forward without pseudoarthrosis. The cortex at the anterior bow thickens, the medullary cavity becomes occluded, and the tibial end atrophies and hardens, presenting an anterior bow shape. After a fracture occurs, conventional treatment fails to achieve local union, leading to pseudoarthrosis formation. Alternatively, due to lack of recognition of this condition, osteotomy may be hastily performed, resulting in nonunion. Continued progression causes absorption of both ends, sclerosis of the bone ends, and further atrophy and tapering of the distal end into a pencil-point shape (Figure 4).
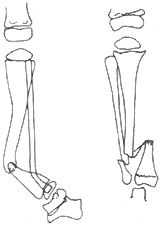
Figure 4: Congenital Tibia Pseudoarthrosis
(2) Cystic Type: After birth, cystic changes appear in the middle to lower third of the tibia, but the diaphysis does not thin, making it clinically inconspicuous. Minor trauma may cause a fracture, followed by nonunion and subsequent pseudoarthrosis formation.
(3) Pseudoarthrosis Type: A defect in the middle to lower tibial segment is noted at birth, forming a pseudoarthrosis. The pseudoarthrosis site may have dense fibrous tissue or cartilage bridging. The bone ends become thinner and atrophic with growth, with the distal end more markedly tapered into a pencil-point shape and the cortex extremely thin. Sometimes, surrounding soft tissues, including the gastrocnemius, also atrophy. If the fibula is involved, similar changes occur.
bubble_chart DiagnosisThe treatment of congenital tibia pseudoarthrosis presents certain difficulties, as fracture healing cannot be achieved without bone grafting. However, conventional bone grafting procedures yield low healing rates, and repeated surgeries further compromise local blood circulation and nutritional conditions. Even if temporary healing occurs, there remains a risk of recurrent fracture. True healing is only considered achieved when no pseudoarthrosis forms by the time of adolescence. If lower limb shortening deformities or foot deformities persist at this stage, additional corrective surgeries are required.
**Treatment Principles** The treatment principles are as follows: ① Once diagnosed, bone grafting should be performed as early as possible, unless systemic or local contraindications exist. In such cases, temporary bracing and gypsum immobilization should be used to protect the affected limb and prevent severe bone resorption leading to aggravated deformity. ② Autologous cancellous bone is the optimal graft material, followed by grafts from direct relatives. Bone bank grafts should be avoided. ③ The scar tissue between bone ends, along with thickened periosteum and sclerotic bone, should be completely excised to improve local blood circulation. ④ Sufficient cancellous bone should be implanted with tight contact. Internal fixation must be robust, external fixation must be secure, and immobilization should be prolonged until bony union is evident before weight-bearing is allowed. ⑤ After pseudoarthrosis fusion, follow-up should be conducted every 6–12 months. Reliable healing is confirmed by gradual disappearance of sclerosis at the bone ends, normalized bone density, patent medullary cavity, and thickening of the diaphysis. If follow-up reveals medullary cavity obstruction, increased sclerosis, or thinning of the diaphysis—indicating impending refracture—repeat bone grafting is necessary.
**(1) Dual Onlay Bone Grafting (Boyd Method)** This technique involves bilateral cortical onlay grafting, providing robust fixation and good alignment while maintaining adequate tibial width. The space between the grafts is densely packed with cancellous bone to ensure tight contact and prevent scar tissue compression. If one graft is resorbed, the contralateral graft remains, offering a reasonable healing rate.
During surgery, the thickened periosteum and interosseous scar tissue are completely excised, avoiding injury to the anterior tibial vessels. The sclerotic and irregular bone ends are resected, and the medullary cavity is reamed. Cortical layers are chiseled from the medial and lateral aspects of the proximal and distal tibia to accommodate the onlay grafts, ensuring tibial length is preserved. Due to the shorter distal segment, the graft may extend to the epiphyseal region. The graft size (length and width) is determined by the patient's tibial dimensions, ideally harvested from the contralateral tibia or a parent’s bone. Fixation screws should avoid the pseudoarthrosis site. The space between the grafts is densely packed with cancellous bone to restore the tibial circumference. Post-suturing removal, a well-fitted long-leg gypsum is applied for six months. If healing occurs, bracing should continue until post-puberty. If signs of recurrent pseudoarthrosis appear during follow-up, prompt repeat grafting is indicated (Figure 1).
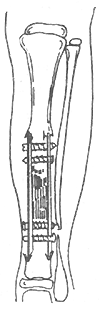
**Figure 1** Bilateral extramedullary onlay bone grafting method
**(2) Bypass Bone Grafting (McFarland Method)** This approach is suitable for angulated or bowed tibias, including cases where fractures are imminent. Instead of direct correction or osteotomy at the pseudoarthrosis site, a long cortical strut graft is placed posteriorly, acting like a bowstring. Weight-bearing forces then bypass the angulated tibial segment, transferring through the graft.
A longitudinal incision is made along the medial tibia, centered on the pseudoarthrosis. Bone slots are created along the posterior tibial border at both ends to accommodate the strut graft, which may be autologous or from a direct relative. The graft should align with the tibial axis, slightly distracting the tibia. Cancellous bone is packed between the graft and the tibia to promote fusion. Postoperatively, a long-leg gypsum is applied, and close follow-up is essential (Figure 2).
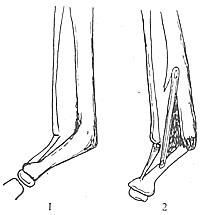
Figure 2 Short-circuit bone grafting
1. Preoperative 2. Post bone grafting
(3) Intramedullary nail fixation and bone grafting The characteristic is that the intramedullary nail passes through both fracture ends, providing stable internal fixation, which is particularly suitable for the atrophic, pencil-tip-shaped distal tibia (Figure 3).

Figure 3 Intramedullary nail fixation with bone grafting
After exposing the tibial fracture ends of the affected child, the scar tissue and thickened bone membrane between the fracture ends are excised, along with the sclerotic bone at the fracture ends, and the medullary cavity is reamed. An intramedullary nail or Steinmann pin is inserted retrograde from the calcaneus or talus into the tibial medullary cavity to maintain the length of the tibia and ensure alignment. The gap between the two fracture ends is filled with a large amount of cancellous bone, tightly packed around the intramedullary nail and tibial fracture ends to restore the original width and circumference of the tibia. Insufficient bone graft volume or loose packing leading to absorption is a cause of surgical failure.
Long-leg gypsum fixation should be properly applied. Follow-up showing initial healing allows the intramedullary nail to be removed from the sole, with continued gypsum fixation until complete tibial healing.
(4) Vascularized autologous fibula grafting A viable bone graft with normal bone membrane and rich blood supply can directly promote healing, with a high success rate. The transplanted fibula, being a longer segment, ensures thorough removal of pathological tissue between the tibial fracture ends and adequate filling of the gap. After survival, the transplanted fibula gradually thickens with body growth, matching the tibial circumference, providing good bone strength and allowing early weight-bearing. This represents a new approach in bone grafting therapy using microsurgical techniques.
Surgical method: Since the affected limb often has undergone multiple surgeries, care must be taken with skin flap blood supply during incision. It is advisable to enter through the original incision, excise scars, and remove sclerotic bone tissue from both tibial fracture ends sufficiently. The remaining gap should be surrounded by normal muscle tissue and subcutaneous fat, with the anterior tibial artery and vein or great saphenous vein dissected and prepared for use. A longitudinal skin incision is made on the upper-middle lateral side of the healthy calf to expose and protect the common peroneal nerve. Enter between the peroneus longus and brevis muscles and the soleus muscle, detach the soleus muscle near the fibula, and retract it medially. The peroneal artery and vein are identified proximally in the incision. The origins of the peroneus longus and brevis muscles are detached along the upper fibula while preserving the bone membrane and a thin layer of muscle tissue. The required length of fibula (the length of the tibial defect after resection plus the length needed for bone end fixation) is harvested from below the fibular neck. The peroneal artery and vein are ligated and cut at the distal osteotomy plane. After releasing the tourniquet, the medullary cavity of the free fibula segment and attached muscle should show bleeding, indicating good blood supply. The fibula vascular pedicle is harvested as long as possible and transferred to the affected vascular area. The distal end of the free fibula is inserted into the distal tibial medullary cavity, while the proximal tibial cortex is partially removed to allow the proximal end of the free fibula to be inlaid and fixed with screws, avoiding intramedullary nail fixation to prevent damage to the medullary nutrient vessels of the free fibula segment. The peroneal artery and vein are anastomosed end-to-end with the anterior tibial artery and vein or great saphenous vein. After blood circulation is reestablished, the thin muscle layer on the free fibula should show bleeding, and the venous anastomosis should exhibit continuous blood flow. Postoperatively, long-leg gypsum splints are applied with the knee flexed at 30°, typically for 3 months. Follow-up X-rays can assess whether the grafted fibula density increases or is absorbed and whether new bone forms at the tibial junction. Isotope scanning or stirred pulse angiography can confirm the blood supply to the grafted segment. Generally, gypsum can be removed after about 3 months when bone healing is achieved, and walking exercises can begin. Short-term outcomes are favorable, and long-term prognosis is optimistic.






