| disease | Pulmonary Edema |
| alias | Pulmonary Edema |
The normal anatomical and physiological mechanisms within the lungs maintain a constant interstitial fluid balance and optimal alveolar moisture, facilitating the performance of various pulmonary functions. If certain factors cause an excessive accumulation of extravascular fluid in the lungs, even to the point of infiltrating the alveoli, it can transition into a pathological state known as pulmonary edema. Clinical manifestations include dyspnea, cyanosis, cough, expectoration of white or blood-tinged frothy sputum, scattered moist rales in both lungs, and imaging findings showing butterfly-shaped or patchy opacities centered around the hilar region. The prognosis of this condition is closely related to the underlying disease, the severity of pulmonary edema, the presence of complications, and the appropriateness of treatment, with significant individual variability.
bubble_chart Etiology
According to the initiating mechanisms, pulmonary disease with edema can be classified into the following categories (Table 1)
Table 1 Classification of Pulmonary Disease with Edema Causes
| I. Alterations in Starling Forces Balance |
| Increased Microvascular Hydrostatic Pressure: ① Elevated pulmonary venous pressure without left heart failure; ② Pulmonary venous pressure elevation secondary to left heart failure; ③ Increased pulmonary microvascular hydrostatic pressure secondary to elevated pulmonary stirred pulse pressure |
| Decreased Plasma Colloid Osmotic Pressure: Hypoalbuminemia |
| Increased Negative Pressure Around Microvessels: ① Application of high negative pressure for drainage in treating pneumothorax or pleural effusion; ② Acute airway obstruction and increased end-expiratory lung volume leading to excessive negative pressure in the pleural membrane cavity (asthma) |
| II. Changes in Alveolar-Capillary Membrane Permeability |
| Pulmonary Infections: Bacteria, viruses, and Chinese Taxillus Herb parasites |
| Inhalation of Toxic Gases: Nitrogen dioxide, ozone, ammonia, chlorine, aeration, etc. |
| Circulating Foreign Substances: Snake venom, bacterial endotoxins, etc. |
| Aspiration of Acidic Gastric Fluid |
| Acute Radiation Pneumonitis |
| Endogenous Vasoconstrictors: Histamine, kinins, etc. |
| Acute Hemorrhagic Pancreatitis |
| III. Lymphatic Drainage Obstruction |
| Lung Transplantation; Lymphatic Cancer; Fibrosing Lymphangitis |
| IV. Unknown or Not Fully Understood Causes |
| High-Altitude Pulmonary Edema; Neurogenic Pulmonary Edema; Anesthetic Overdose; Pulmonary Embolism; Convulsions |
[Anatomical Basis of Pulmonary Edema]
The alveolar surface is lined by epithelial cells, with approximately 90% covered by flat type I alveolar cells and fewer type II alveolar cells. These alveolar epithelial cells are tightly arranged, preventing fluid passage under normal conditions. Type II alveolar cells are rich in phospholipids, primarily dipalmitoyl phosphatidylcholine, whose secretions enter the alveoli to form a thin layer of surfactant that reduces alveolar surface tension, maintains alveolar expansion, and prevents interstitial fluid from leaking into the alveolar cavity. Pulmonary capillaries are lined with thin, flat endothelial cells, whose junctions are relatively loose, allowing small amounts of fluid and certain protein particles to pass through.
Electron microscopy reveals that the basement membrane between alveolar epithelium and vascular endothelium is not fully fused, with the alveolar wall adjacent to capillaries exhibiting a thinner and a thicker side (as shown in Figure 1). The thin side consists of fused basement membranes of the alveolar epithelium and capillary endothelium, forming a three-layered structure (alveolar epithelium, basement membrane, and capillary endothelium) that facilitates gas exchange between blood and alveoli. The thick side comprises the capillary endothelial layer, basement membrane, a network of collagen and elastic fibers, alveolar epithelium, a thin fluid layer, and the surfactant layer. The interstitial space (pulmonary interstitium) separates the epithelial and endothelial basement membranes and connects with the peribronchovascular interstitial space, interlobular septa, and subpleural space to facilitate fluid exchange. Fluid entering the pulmonary interstitium is primarily reabsorbed by the lymphatic system. In the thick alveolar septum, receptors composed of nerves and punctate collagen material can be observed under electron microscopy. When interstitial fluid increases, swollen collagen fibers stimulate "J" receptors, transmitting signals to the central nervous system, reflexively increasing respiratory rate and depth, elevating negative intrathoracic pressure, and enhancing lymphatic drainage.
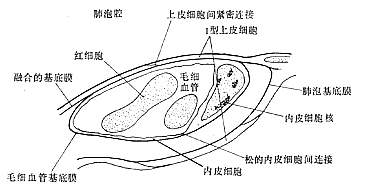
Figure 1 Schematic Diagram of Alveolar Capillary Structure
[Physiological Basis of Pulmonary Edema]
The factors controlling fluid movement across biological semipermeable membranes can be summarized by the Starling equation. When applied to the lungs and accounting for filtration area and fluid reabsorption mechanisms, it can be rewritten as:
EVLW = {(SA × Lp) [(Pmv - Ppmv) - σ(πmv - πpmv)]} - Flymph
Where EVLW is the extravascular lung water content; SA is the filtration area; Lp is the hydraulic conductivity; Pmv and Ppmv are the microvascular and perimicrovascular hydrostatic pressures, respectively; σ is the protein reflection coefficient; πmv and πpmv are the microvascular and perimicrovascular colloid osmotic pressures, respectively; and Flymph represents lymphatic flow, encompassing all mechanisms for fluid reabsorption into the vasculature.
It should be noted that the term "microvessels" rather than "capillaries" is used here because fluid filtration can also occur at pulmonary small stirred pulses and venules. Additionally, SA×Lp=Kf, which is the filtration coefficient of hydraulic conductivity. Although it is difficult to measure SA and Lp, this highlights the importance of SA in the overall fluid balance in the lungs. The reflection coefficient represents the permeability of blood vessels to proteins. If the semipermeable membrane completely prevents the passage of proteins that can generate osmotic pressure, the σ value is 1.0; conversely, if it offers no resistance to protein filtration, the σ value is 0. Thus, the σ value reflects how changes in vascular permeability affect the osmotic pressure gradient, thereby influencing fluid movement between the pulmonary vasculature and the interstitium. The σ value of pulmonary vascular endothelium is 0.9, while that of alveolar epithelium is 1.0. Therefore, to some extent, the endothelium is more prone to fluid filtration than the alveolar epithelium, leading to interstitial edema occurring before alveolar edema.
From the formula, it can be seen that if SA, Lp, Pmv, and πpmvpartially or entirely increase while other factors remain unchanged, EVLW will increase. A decrease in Ppmv, σ, πmv, and Flymph also produces the same effect. Due to the influence of gravity and the mechanical properties of the lungs, Pmv and Ppmv are not uniform across all regions of the lungs. In lung regions below the level of the right atrium, although both Pmv and Ppmv may increase, the rise in Pmv is greater than that in Ppmv, which helps explain why pulmonary edema tends to occur first in areas most affected by gravity.Under normal conditions, despite the influence of posture, gravity, lung volume, and changes in circulating fluid volume on pulmonary microvascular and interstitial hydrostatic pressure, both the pulmonary interstitium and alveoli maintain an ideal state of moisture. This is because the lymphatic system, pulmonary interstitial protein, and compliance characteristics help counteract fluid retention and continuously remove excess fluid from the lungs. When pulmonary vascular hydrostatic pressure and permeability increase, lymphatic flow can increase more than tenfold. A secondary effect is the dilution of interstitial protein, caused by increased fluid filtration due to elevated hydrostatic pressure in the microvasculature, which reduces πpmvand, in turn, decreases net filtration. However, this does not counteract pulmonary edema caused by increased vascular permeability. Another factor preventing pulmonary edema is the compliance change effect. The tightly connected gel structure of the pulmonary interstitium is less deformable and has poor compliance. After grade I interstitial fluid accumulation, pressure rises rapidly, preventing further filtration. However, due to the limited expansion range of the interstitial space, if the rate of interstitial fluid removal cannot keep up with the rate of microvascular filtration, alveolar edema is more likely to occur.
[Pathogenesis of pulmonary edema]
Although the factors affecting fluid exchange across pulmonary blood vessels have been listed above, in reality, pulmonary edema is usually the result of a combination of multiple pathogenic mechanisms. Below is a brief introduction to the pathogenesis of several clinically common types of pulmonary edema.
(1) Pulmonary edema due to elevated pulmonary microvascular hydrostatic pressure: This is commonly seen clinically in left heart failure caused by myocardial infarction, hypertension, and aortic valve regurgitation, as well as in conditions such as mitral stenosis and pulmonary venous occlusive disease that increase pulmonary venous pressure, leading to elevated pulmonary microvascular hydrostatic pressure. It can also dilate previously closed capillary beds, increasing the permeability coefficient. When the fluid filtration caused by these two factors exceeds the lymphatic system's clearance capacity, pulmonary edema occurs.
(2) Pulmonary edema due to increased permeability of microvessels and alveolar walls: Diffuse pulmonary infections, inhalation of toxic gases, and shock (especially Gram-negative bacterial sepsis and hemorrhagic pancreatitis) can damage capillary endothelium and alveolar epithelium, increasing permeability and causing pulmonary edema.
(3) Decreased plasma colloid osmotic pressure: Although liver and kidney diseases can cause hypoalbuminemia and reduce colloid osmotic pressure, pulmonary edema rarely occurs because the colloid osmotic pressure around the microvasculature also decreases. Only when accompanied by elevated hydrostatic pressure in the microvasculature does it induce pulmonary edema.
(4) Impaired pulmonary lymphatic drainage: It is estimated that in steady-state conditions, adult pulmonary lymphatic flow can reach 200 ml/h, making it the most important factor in preventing pulmonary edema. When acute increases in microvascular hydrostatic pressure or permeability occur, pulmonary lymphatic flow can increase more than tenfold, slowing the rate of pulmonary edema formation. If drainage is obstructed or stagnant, it can induce interstitial or even alveolar edema.
(5) Re-expansion pulmonary edema When the speed of thoracentesis for air or fluid removal is too fast or the volume is excessive, it can abruptly increase the negative pressure in the pleural cavity, reducing the hydrostatic pressure around the microvessels and increasing the filtration pressure difference. Simultaneously, due to the excessive negative pleural pressure, both the number of open pulmonary capillaries and the inflow blood volume increase, thereby expanding the filtration area and filtration coefficient. Additionally, after lung tissue collapses, the production of surfactant decreases, lowering the protein reflection coefficient of the alveolar epithelium and inducing the formation of alveolar edema.
(6) High-altitude pulmonary edema is prone to occur at altitudes above 3,000 meters, with excessive exercise or labor as triggering factors, and is more common in young people under 25 years old. The mechanism is still unclear. It may be related to pulmonary arteriolar spasm or pulmonary venous contraction. The improvement of the patient's condition after oxygen inhalation or returning to the plains suggests the role of hypoxia, but hypoxia itself does not alter the permeability of pulmonary microvessels. Therefore, the increase in cardiac output after exercise and the rise in pulmonary arteriolar pressure, together with hypoxic pulmonary arteriolar constriction, can produce this typical pre-capillary pressure elevation pulmonary edema.
(7) Neurogenic pulmonary edema can occur in patients with central nervous system diseases but without obvious left heart failure. Many studies suggest it is related to sympathetic nervous system activity. The massive release of adrenergic mediators leads to peripheral vasoconstriction, increased blood pressure, and blood transfer into the circulation, while left ventricular compliance may decrease. Both factors elevate left atrial pressure, inducing pulmonary edema. Additionally, stimulation of adrenergic receptors can directly increase capillary permeability, but compared to the pressure elevation, this effect is relatively minor.
bubble_chart Pathological Changes
The lung surface appears pale with increased water content, and a large amount of fluid exudes from the cut surface. Microscopically, it can be divided into the interstitial stage, alveolar wall stage, and alveolar stage.
The interstitial stage is the earliest manifestation of pulmonary edema, where fluid is confined to the loose connective tissue around extra-alveolar blood vessels and conducting airways. The peribronchial and perivascular spaces and interlobular septa widen, and lymphatic vessels dilate. As fluid retention progresses, it enters the alveolar wall stage. Fluid accumulates on one side of the thick alveolar capillary membrane, and the alveolar wall progressively thickens. When it develops into the alveolar stage, the alveolar walls, filled with fluid, lose their ring-like structure and exhibit folds. Whether caused by increased microvascular pressure or permeability, the protein content of the fluid in the alveolar space is the same as that in the pulmonary interstitium, indicating surfactant destruction and loss of epithelial filtration capacity.
The pathophysiological changes of pulmonary edema can affect compliance, diffusion, ventilation/perfusion ratio, and breathing patterns. The severity correlates with the pathological changes described above, being mildest in the interstitial stage and most severe in the alveolar stage. Increased lung water content and surfactant destruction reduce lung compliance and increase respiratory effort. Fluid retention in the interstitium and alveolar walls widens the diffusion distance. Partial or complete filling of alveoli with fluid reduces the diffusion area and lowers the ventilation/perfusion ratio, leading to increased alveolar-arterial oxygen tension difference and hypoxemia. Regional differences in lung compliance cause inhaled gas to preferentially enter alveoli with better compliance, increasing the ventilation/perfusion ratio. Meanwhile, interstitial fluid accumulation stimulates receptors, resulting in rapid, shallow breathing, further increasing minute dead space ventilation, reducing respiratory efficiency, and increasing respiratory effort. When respiratory muscle fatigue prevents compensatory increases in ventilation to maintain alveolar ventilation, CO2 retention and respiratory acidosis occur.
Pulmonary edema can impact hemodynamics even in the interstitial stage. Elevated interstitial hydrostatic pressure compresses nearby microvessels, increasing pulmonary vascular resistance and raising pulmonary artery pressure. Hypoxia and acidosis can directly constrict pulmonary vessels, further worsening hemodynamics, increasing right heart load, and leading to cardiac insufficiency. If not corrected in time, it can result in death due to heart failure or arrhythmias.
bubble_chart Clinical Manifestations
In the interstitial phase of pulmonary edema, patients often experience cough, chest tightness, grade I shallow and rapid breathing. Physical examination may reveal wheezing sounds in both lungs, and signs of heart disease may be detected in cardiogenic pulmonary edema. Both PaO2 and PaCO2 show grade I reduction. When pulmonary edema fluid infiltrates the alveoli, patients may present with pale complexion, cyanosis, severe dyspnea, and cough with large amounts of white or bloody frothy sputum, along with widespread moist rales in both lungs. Blood gas analysis indicates worsening hypoxemia, and may even show CO2 retention and mixed acidosis.
bubble_chart Auxiliary Examination
During the interstitial phase of pulmonary edema, the main X-ray findings include blurred and increased pulmonary vascular markings, indistinct hilar shadows, reduced lung translucency, and widened interlobular septa. Kerley B lines, which are transverse lines perpendicular to the pleura, can be seen in the lower lung fields near the costophrenic angles. Occasionally, Kerley A lines—longer, arc-shaped lines extending obliquely toward the hilum—may appear in the upper lungs. Alveolar edema primarily manifests as acinar-shaped dense shadows, appearing as irregular, coalescing, blurred shadows that are either diffusely distributed or localized to one side or lobe, or extend outward from both hila, gradually fading into the classic butterfly-shaped shadow. Sometimes, a small amount of pleural effusion may accompany these findings. However, these manifestations only appear when lung fluid content increases by more than 30%. CT and MRI can quantify and even differentiate between pulmonary congestion and interstitial edema, but these methods are costly.
bubble_chart Treatment Measures
(1) Disease Cause Treatment is crucial for the prognosis of pulmonary edema, as it can alleviate or correct the disorder of fluid exchange inside and outside the pulmonary blood vessels. For those with excessive infusion speed, the infusion should be stopped or slowed immediately. Dialysis can be used for uremia patients. For those induced by infection, appropriate antibiotics should be administered immediately. For those who have inhaled toxic gases, they should be removed from the scene immediately and given detoxifying agents. For those who have overdosed on anesthetics, gastric lavage and antidotes should be administered immediately.
(2) Morphine A dose of 5–10 mg administered subcutaneously or intravenously can alleviate anxiety and reduce peripheral vascular resistance through central sympathetic inhibition, thereby shifting blood from the pulmonary circulation to the systemic circulation. It also relaxes airway smooth muscles, improving ventilation. It is most effective for cardiogenic pulmonary edema but is contraindicated in cases of shock, respiratory depression, and chronic obstructive pulmonary disease complicated by pulmonary edema.
(3) Diuretics Intravenous injection of furosemide (40–100 mg) or bumetanide (1 mg) can rapidly induce diuresis, reduce circulating blood volume, increase plasma colloid osmotic pressure, and decrease microvascular filtration of fluid. Additionally, intravenous furosemide can dilate veins, reduce venous return, and alleviate pulmonary edema even before the diuretic effect takes place. However, it should not be used in cases of insufficient blood volume.
(4) Oxygen Therapy Patients with pulmonary edema usually require high-concentration oxygen inhalation to improve hypoxemia, preferably via a face mask. A humidifier containing 75–95% alcohol or 10% silicone can help eliminate foam. For cases where hypoxemia is difficult to correct, a ventilator can be used to deliver oxygen via a face mask or artificial airway, which helps increase interstitial hydrostatic pressure, reduce cardiac output, and lower microvascular hydrostatic pressure, thereby decreasing fluid filtration out of the blood vessels. However, this is contraindicated in cases of insufficient cardiac output.
(5) Vasodilators Intravenous infusion of sodium nitroprusside (15–30 μg/min) can dilate small arteries and venules. Alpha-receptor blockers can counteract the vasoconstrictive effects of catecholamines, histamine, and serotonin, dilating small arteries and venules in both pulmonary and systemic circulation. Both can reduce cardiac preload and afterload, decrease pulmonary circulation blood flow and microvascular hydrostatic pressure, thereby alleviating pulmonary edema. Commonly used agents include phentolamine (0.2–1 mg/min) or phenoxybenzamine (0.5–1 mg/kg) via intravenous infusion. However, the infusion rate should be adjusted, and blood volume replenished to maintain arterial blood pressure within the normal range.
(6) Cardiotonics These are mainly indicated for pulmonary edema induced by rapid atrial fibrillation or flutter. For patients who have not used digitalis drugs within the past two weeks, strophanthin K (0.25 mg) or lanatoside C (0.4–0.8 mg) can be slowly injected intravenously after dissolving in glucose.
(7) Aminophylline Intravenous injection of aminophylline (0.25 g) can effectively dilate bronchi, improve myocardial contractility, increase renal blood flow, and promote sodium excretion. However, the injection speed should be monitored to prevent adverse cardiac effects.
(8) Adrenal Glucocorticoids The therapeutic value of glucocorticoids for pulmonary edema remains controversial. Some studies suggest they can reduce inflammatory responses, decrease microvascular permeability, promote surfactant synthesis, enhance myocardial contractility, lower peripheral vascular resistance, and stabilize lysosomal membranes. They can be used for high-altitude pulmonary edema, toxic pulmonary edema, and myocarditis complicated by pulmonary edema. Typically, dexamethasone (20–40 mg/day) or hydrocortisone (400–800 mg/day) is administered intravenously for 2–3 days.
(9) Reducing Pulmonary Circulatory Blood Volume Placing the patient in a sitting position with legs dangling or applying venous tourniquets to the limbs in rotation (releasing one limb every 20 minutes for 5 minutes) can reduce venous return. This is suitable for fluid overload or cardiogenic pulmonary edema but is contraindicated in cases of shock and anemia.
A definitive diagnosis of pulmonary edema can often be made based on medical history, symptoms, physical examination, and X-ray findings. However, since significant X-ray changes only appear when lung water content increases by more than 30%, CT and magnetic resonance imaging may be used when necessary to aid in early diagnosis and differential diagnosis. The thermal dilution method and plasma colloid osmotic pressure-pulmonary capillary wedge pressure gradient measurement can calculate extravascular lung water content and determine the presence of pulmonary edema, but both require the placement of a pulmonary artery catheter, making them invasive procedures. When performing lung perfusion scans using 99mTc-labeled human serum albumin or 113mIn-transferrin, if vascular permeability is increased, these tracers may accumulate in the lung interstitium, particularly in cases of permeability pulmonary edema. Additionally, the management of cardiogenic and non-cardiogenic pulmonary edema differs, and the two should be distinguished (Table 2).
Table 2. Differentiation Between Cardiogenic and Non-cardiogenic Pulmonary Edema
Item | Cardiogenic Pulmonary Edema | Non-cardiogenic Pulmonary Edema |
| Medical History | History of heart disease | No history of heart disease, but with other underlying diseases |
| Signs | Signs of heart disease | No abnormal cardiac signs |
| X-ray Findings | Butterfly-shaped infiltration radiating from the hila, increased vascular shadows in the upper lung fields | Normal hila, diffuse small patchy shadows in the peripheral lungs |
| Edema Fluid Characteristics | Low protein content | High protein content |
| Edema Fluid Colloid Osmotic Pressure/Blood Colloid Osmotic Pressure | <60% | >75% |
| Pulmonary Capillary Wedge Pressure | >1.3kPa | <1.3kPa |
| Pulmonary Artery Diastolic Pressure-Pulmonary Capillary Wedge Pressure Gradient | <0.6kPa | >0.6kPa |




