| disease | Gastrointestinal Bleeding |
Gastrointestinal bleeding is a common and serious clinical symptom. The gastrointestinal tract refers to the passage from the esophagus to the anus, including the stomach, duodenum, jejunum, ileum, cecum, colon, and rectum. Upper gastrointestinal bleeding refers to bleeding in the esophagus, stomach, duodenum, upper jejunum, as well as the pancreatic and bile ducts above the ligament of Treitz. Intestinal bleeding below the ligament of Treitz is referred to as lower gastrointestinal bleeding.
bubble_chart Etiology
Gastrointestinal bleeding can be caused by inflammation, mechanical {|###|} injury, vascular lesions, tumors, and other factors in the digestive tract itself, or by diseases of adjacent organs and systemic diseases affecting the digestive tract.
(1) Disease causes of upper gastrointestinal bleeding
1. Esophageal diseases: esophagitis (reflux esophagitis, esophageal diverticulitis), esophageal cancer, esophageal ulcer, Mallory-Weiss syndrome, injury caused by instrumental examination or foreign bodies, radiation injury, and chemical injury caused by strong acids or alkalis.
2. Gastric and duodenal diseases: peptic ulcer, acute or chronic gastritis (including drug-induced gastritis), gastric mucosal prolapse, stomach cancer, acute gastric dilatation, duodenitis, remnant gastritis, remnant gastric ulcer or cancer. Also include lymphoma, leiomyoma, polyp, fleshy tumor, hemangioma, neurofibroma, diaphragmatic hernia, gastric volvulus, diverticulitis, hookworm disease, etc.
3. Jejunal ulcer and anastomotic ulcer after gastroenterostomy.
4. Portal hypertension, bleeding from esophageal and gastric varices, portal hypertensive gastropathy, cirrhosis, portal vein obstruction due to portal vein inflammation or thrombosis, hepatic vein obstruction (Budd-Chiari syndrome).
5. Diseases of adjacent organs or tissues to the upper gastrointestinal tract(1) Biliary tract bleeding: bile duct or gallbladder stones, biliary ascariasis, gallbladder or bile duct diseases, liver cancer, liver abscess, or rupture of hepatic vascular lesions.
(2) Pancreatic diseases involving the duodenum: pancreatic abscess, pancreatitis, pancreatic cancer, etc.
(3) Thoracic or abdominal aortic aneurysm rupturing into the digestive tract.
(4) Mediastinal tumor or abscess rupturing into the esophagus.
6. Systemic diseases manifesting as gastrointestinal bleeding
(1) Hematologic diseases: leukemia, aplastic anemia, hemophilia, etc.
(2) Uremia.
(3) Connective tissue diseases: vasculitis.
(4) Stress ulcer: severe infection, surgery, trauma, shock, glucocorticoid therapy, and stress states caused by certain diseases, such as cerebrovascular accidents, pulmonary heart disease, severe heart failure, etc.
(5) Acute infectious diseases: epidemic hemorrhagic fever, leptospirosis.
(2) Disease causes of lower gastrointestinal bleeding
1. Anal diseases: hemorrhoids, anal fissure, anal fistula.
2. Rectal diseases: rectal injury, nonspecific proctitis, subcutaneous nodular proctitis, rectal tumors, rectal carcinoid, adjacent malignant tumors or abscesses invading the rectum.
3. Colonic diseases: bacterial dysentery, amebic dysentery, chronic nonspecific ulcerative colitis, diverticula, polyps, cancer, and vascular malformations.
4. Small intestine diseases: acute hemorrhagic necrotizing enteritis, intestinal tuberculosis, Crohn's disease, jejunal diverticulitis or ulcer, intussusception, small intestine tumors, gastrointestinal stromal tumors, small intestine hemangioma, and vascular malformations.
bubble_chart Clinical ManifestationsThe clinical manifestations of gastrointestinal bleeding depend on the nature, location, amount, and speed of the {|###|}blood loss, as well as the patient's age, cardiac and renal function, and overall systemic condition.
(1) Bleeding patterns Acute massive bleeding often presents as hematemesis; chronic minor bleeding is manifested by positive fecal occult blood. When the bleeding site is above the ligament of Treitz in the jejunum, the clinical presentation is hematemesis. If the blood remains in the stomach for an extended period, it turns into acidic hemoglobin due to gastric acid action, appearing coffee-bean-colored. If the bleeding is rapid and voluminous, the hematemesis will be bright red. Melena or tarry stools indicate bleeding in the upper gastrointestinal tract. However, if bleeding from a duodenal lesion is too rapid, the stool color may turn purplish-red due to the short transit time in the intestines. Bleeding in the right colon results in bright red stools. Minor oozing from lesions in the ileum or right colon can also cause melena.
(2) Peripheral circulatory failure due to blood loss Massive upper gastrointestinal bleeding leads to acute peripheral circulatory failure. A large amount of blood loss, persistent bleeding, or inadequate treatment can reduce tissue perfusion and cause cellular hypoxia. This, in turn, can lead to peripheral vasodilation due to hypoxia, metabolic acidosis, and the accumulation of metabolic byproducts, as well as widespread capillary damage. Consequently, a significant amount of fluid may stagnate in the abdominal cavity and surrounding tissues, sharply reducing effective blood volume and severely compromising the blood supply to the heart, brain, and kidneys, ultimately resulting in irreversible shock and death.
During the progression of peripheral circulatory failure due to bleeding, clinical symptoms may include dizziness, palpitation, nausea, thirst, blackout, or syncope. The skin may appear pale and clammy due to vasoconstriction and insufficient blood perfusion. Pressing the nail bed results in prolonged pallor without quick recovery. Venous filling is poor, and superficial veins often collapse. Patients may feel fatigued and weak, progressing to listlessness, dysphoria, and even sluggish responses or confusion. Elderly patients, with their diminished organ reserve function and underlying conditions such as cerebral arteriosclerosis, hypertension, coronary artery disease, and chronic bronchitis, are at higher risk of multi-organ failure and death even with relatively minor bleeding.(3) Azotemia Azotemia can be classified into three types: enterogenic, renal, and prerenal. Enterogenic azotemia refers to the elevation of blood nitrogen levels due to the absorption of protein breakdown products from the intestines after massive upper gastrointestinal bleeding. Prerenal azotemia occurs when peripheral circulatory failure due to blood loss temporarily reduces renal blood flow, glomerular filtration rate, and renal excretory function, leading to nitrogen retention. After correcting hypotension and shock, blood urea nitrogen levels can quickly return to normal. Renal azotemia results from severe and prolonged shock causing renal tubular necrosis (acute renal failure) or exacerbating pre-existing renal damage due to blood loss. Clinically, oliguria or anuria may occur. If bleeding stops, azotemia may persist for more than four days, and blood urea nitrogen may not normalize despite adequate fluid resuscitation and shock correction.
(4) Fever Most patients develop a low-grade fever within 24 hours after massive bleeding. The fever may be caused by reduced blood volume, anemia, peripheral circulatory failure, or the absorption of blood protein breakdown products, leading to dysfunction of the thermoregulatory center. When analyzing the cause of fever, other factors such as concurrent pneumonia should also be considered.
(5) Compensatory Function After Hemorrhage When the amount of gastrointestinal bleeding exceeds one-fourth of the blood volume, cardiac output and diastolic blood pressure significantly decrease. At this time, the body releases a large amount of catecholamines correspondingly, increasing peripheral vascular resistance and heart rate to maintain blood perfusion in various organs. In addition to cardiovascular responses, hormonal secretion and the hematopoietic system also compensate accordingly. The secretion of aldosterone and posterior pituitary hormones increases to minimize the loss of interstitial fluid and restore or maintain blood volume. If compensation is still insufficient, the hematopoietic system is stimulated, leading to active proliferation of blood cells and an increase in red blood cells and reticulocytes.
(1) Early identification of massive upper gastrointestinal bleeding If the signs of acute peripheral circulatory failure caused by upper gastrointestinal bleeding appear before hematemesis and melena, it must be differentiated from toxic shock, allergic shock, cardiogenic shock, acute hemorrhagic necrotizing pancreatitis, as well as other hemorrhagic shocks caused by conditions such as ruptured ectopic pregnancy, spontaneous or traumatic splenic rupture, or ruptured aneurysm. Sometimes, upper gastrointestinal endoscopy and rectal examination are necessary to detect blood that has not yet been vomited or passed in the stool, allowing for early diagnosis.
Hematemesis and melena caused by upper gastrointestinal bleeding should first be distinguished from those caused by swallowing blood due to epistaxis, tooth extraction, or tonsillectomy. They should also be differentiated from hemoptysis caused by pulmonary tuberculosis, bronchiectasis, bronchial lung cancer, or mitral stenosis. Additionally, oral intake of animal blood, charcoal, bismuth preparations, or certain Chinese medicinals can also cause black stools, which sometimes need to be distinguished from melena caused by upper gastrointestinal bleeding.
(2) Estimation of blood loss When upper gastrointestinal bleeding reaches about 20ml, the fecal occult blood (guaiac) test may show a positive reaction. When the blood loss exceeds 50–70ml, melena may appear. Severe bleeding refers to cases where 1500ml of blood transfusion is required within 3 hours to correct shock. Severe bleeding can be further classified as massive bleeding (requiring 300ml of blood transfusion per hour to stabilize blood pressure) or major hemorrhage (hemoglobin still dropping below 10g/dl after a 1000ml blood transfusion). Persistent bleeding refers to active bleeding observed twice within 24 hours via gastroscopy, lasting more than 60 hours and requiring 3000ml of blood transfusion to stabilize circulation. Recurrent bleeding refers to two episodes of bleeding separated by at least 1–7 days. If the blood loss does not exceed 400ml, the grade I reduction in blood volume can be quickly compensated by tissue fluid. However, if the blood loss exceeds 500ml and occurs rapidly, the patient may experience dizziness, weakness, tachycardia, and low blood pressure. As blood loss increases, symptoms become more pronounced and may even lead to hemorrhagic shock.
The estimation of upper gastrointestinal blood loss is primarily based on the clinical manifestations of peripheral circulatory failure caused by reduced blood volume, especially dynamic observations of blood pressure and pulse. The degree of blood loss can also be estimated based on the patient's red blood cell count, hemoglobin level, and hematocrit.
(3) Diagnosis of the cause and location of bleeding
1. Medical history and signs 80–90% of patients with peptic ulcers have a long history of regular epigastric pain, and bleeding may occur due to triggers such as improper diet or mental fatigue. Pain often lessens after bleeding, and emergency or early gastroscopy can reveal the ulcer bleeding site. A history of chronic hepatitis or schistosomiasis, accompanied by liver palms, spider angiomas, abdominal wall varices, splenomegaly, or ascites, suggests bleeding from esophageal varices due to portal hypertension. In patients over 45 years old with chronic positive fecal occult blood tests and iron-deficiency anemia, stomach cancer or hiatal hernia should be considered. A history of taking anti-inflammatory painkillers or corticosteroids, or severe trauma, surgery, or sepsis, suggests stress ulcers or acute gastric mucosal lesions. Unexplained intestinal obstruction and hematochezia in patients over 50 should raise suspicion of colon tumors. In patients over 60 with a history of coronary heart disease or atrial fibrillation, abdominal pain and hematochezia suggest ischemic bowel disease. Sudden abdominal pain, shock, and hematochezia should immediately raise suspicion of ruptured aneurysm. Jaundice, fever, and abdominal pain accompanied by gastrointestinal bleeding cannot exclude biliary tract bleeding, commonly seen in bile duct stones or ascariasis.
2. Special Diagnostic Methods In recent years, there has been significant progress in the clinical research of gastrointestinal bleeding. In addition to the traditional methods—X-ray barium meal or enema examination—endoscopy has become widely used. On the basis of diagnosis, therapeutic interventions for bleeding have also been developed.
(1) X-ray barium examination: Only applicable to patients whose bleeding has stopped and whose condition is stable. Its positive rate for diagnosing the cause of acute gastrointestinal bleeding is not high.
(2) Endoscopy
(3) Angiography
(4) Radionuclide imaging: In recent years, radionuclide imaging has been used to identify sites of active bleeding. The method involves intravenous injection of 99m technetium colloid, followed by abdominal scanning to detect evidence of tracer extravasation from blood vessels, providing preliminary localization.
bubble_chart Treatment Measures
(1) General Treatment Bed rest; observe the patient's complexion and whether the skin of the limbs is cold and clammy or warm; record blood pressure, pulse, amount of bleeding, and hourly urine output; maintain venous access and measure central venous pressure. Keep the patient's airway clear to avoid choking during hematemesis. Patients with massive bleeding should fast, while those with minor bleeding may consume appropriate fluids. Most patients often develop fever after bleeding, and antibiotics are generally unnecessary.
(2) Blood Volume Replacement When hemoglobin falls below 9g/dl or systolic blood pressure is below 12kPa (90mmHg), an adequate amount of whole blood should be transfused immediately. For patients with cirrhosis and portal hypertension, caution is needed to prevent the transfusion from increasing portal venous pressure and triggering rebleeding. Avoid excessive transfusion or fluid infusion, which may lead to acute pulmonary edema or induce rebleeding.
(3) Hemostatic Management for Massive Upper Gastrointestinal Bleeding
1. Gastric Cooling Repeatedly irrigate the gastric cavity with 10–14°C ice water via a gastric tube to cool the stomach. This causes vasoconstriction, reduces blood flow, and inhibits gastric secretion and digestion. It also weakens fibrinolytic activity at the bleeding site, thereby achieving hemostasis.
2. Oral Hemostatic Agents For bleeding from peptic ulcers, which involves mucosal lesions, vasoconstrictors such as norepinephrine (8mg in 150ml of ice-cold saline) can be administered orally in divided doses. This induces strong contraction of small bleeding vessels to stop bleeding. This method is not recommended for elderly patients.
3. Inhibiting Gastric Acid Secretion and Protecting Gastric Mucosa H2 receptor antagonists like cimetidine inhibit gastric acid and raise gastric pH, thereby reducing H+ back-diffusion and promoting hemostasis. They are effective in preventing and treating stress ulcers and acute gastric mucosal lesions. In recent years, proton pump inhibitors like omeprazole, which block H+, K+ATPase, have been used. For massive bleeding, 40mg can be administered intravenously as a single dose.
4. Endoscopic Hemostasis Topical application of 5% Monsell's solution (basic ferric sulfate solution) induces local gastric wall spasm and vasoconstriction around the bleeding site, promoting blood coagulation and achieving hemostasis. High-frequency electrocoagulation under endoscopic visualization is suitable for persistent bleeding. However, precise coagulation of the bleeding point is challenging, and direct contact with the bleeding surface may cause temporary bleeding. In recent years, endoscopic laser therapy has been widely adopted, causing tissue protein coagulation and small vessel constriction, leading to immediate mechanical vascular occlusion or intravascular thrombosis.
5. Non-Surgical Treatment for Esophageal Variceal Bleeding
(1) Balloon Tamponade: An effective but temporary non-surgical method to control bleeding. For half a century, this has been the first-line treatment for massive esophageal variceal bleeding, with an immediate hemostasis rate of 90%. Complications of balloon tamponade include: ① Airway obstruction and suffocation; ② Esophageal wall ischemia, necrosis, and rupture; ③ Aspiration pneumonia. In recent years, the balloon has been modified to allow a thin fiberoptic endoscope to pass through the central lumen, enabling direct observation of variceal bleeding and the effectiveness of tamponade.
(2) Drug therapy to reduce portal pressure: It decreases blood flow at the bleeding site, providing conditions for the coagulation process, thereby achieving hemostasis. This method is effective not only for variceal rupture bleeding but also for ulcers, erosions, and mucosal tears. Two types of drugs can be selected: vasoconstrictors and vasodilators. ① Vasopressin and its derivatives: Posterior pituitary extract is the most commonly used, with a dose of 0.4 U/min administered via continuous intravenous infusion. After hemostasis is achieved, the dose is reduced by 0.1 U/min every 12 hours. It can reduce portal pressure by 8.5%, with a hemostasis success rate of 50–70%. However, the recurrence rate of bleeding is high, and the drug itself may cause severe complications such as thrombosis in the portal venous system and coronary artery vasoconstriction. It should be used in combination with nitroglycerin. Derivatives of this drug include octapressin and terlipressin. ② Somatostatin and its derivatives: In recent years, octreotide (Sandostatin) has been synthesized, which can reduce main portal blood flow by 25–35% and lower portal pressure by 12.5–16.7%. It also simultaneously causes visceral vasoconstriction and inhibits gastrin and gastric acid secretion. It is suitable for bleeding from esophageal varices in cirrhosis, with a hemostasis success rate of 70–87%. For peptic ulcer bleeding, the hemostasis efficiency is 87–100%. Administer 100 μg via slow intravenous injection, followed by a continuous infusion of 25 μg per hour. ③ Vasodilators: These are not recommended during massive bleeding but are considered more suitable for use in combination with vasoconstrictors or for preventing rebleeding after hemostasis. Commonly used vasodilators include nifedipine and nitrates such as nitroglycerin, which have the effect of reducing portal pressure.
(IV) Management of Lower Gastrointestinal Bleeding The fundamental measures include blood transfusion, fluid infusion, and correction of hypovolemic shock caused by insufficient blood volume. First, it is essential to rule out the possibility of upper gastrointestinal bleeding as much as possible. Then, targeted treatment should be administered based on the localization and disease cause diagnosis of lower gastrointestinal bleeding, as outlined in Table 1.
Table 1: Steps for Managing Lower Gastrointestinal Bleeding
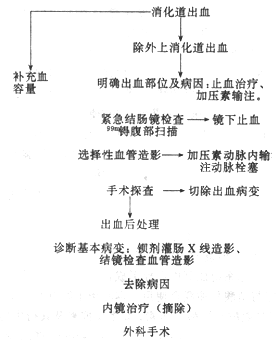
Endoscopic hemostasis is the preferred method for treating lower gastrointestinal bleeding. Local treatments include spraying 5% Monsel's solution, norepinephrine, or thrombin complex. Electrocautery and laser therapy may also be employed.
(V) Surgical Management
1. **Esophagogastric Variceal Bleeding** If non-surgical treatments such as blood transfusion, hemostatic drugs, balloon tamponade (e.g., Sengstaken-Blakemore tube), sclerotherapy, or embolization fail to control bleeding, emergency variceal ligation should be performed. Although this method can achieve hemostasis, the recurrence rate of bleeding is relatively high. Combining splenorenal shunt surgery may reduce the recurrence rate. Other surgical options include portoazygous disconnection, H-shaped mesenteric-caval shunt, and splenocaval shunt, which are also used clinically. Elective portocaval shunt surgery has a low mortality rate and preventive significance. Liver transplantation may also be considered for cases caused by severe cirrhosis.
2. **Ulcer Bleeding** Surgical intervention is warranted under the following conditions: - Persistent upper gastrointestinal bleeding for more than 48 hours without cessation. - Failure to correct blood volume and unstable blood pressure despite transfusing 1500ml of blood within 24 hours. - Recurrent bleeding during conservative treatment. - Active bleeding with visible pulsatile vessels observed during endoscopy. The mortality rate in such cases can be as high as 30%, necessitating early surgical intervention.
3. **Superior Mesenteric Artery Thrombosis or Embolism** This condition commonly occurs in middle-aged and elderly individuals with atherosclerosis, presenting as sudden abdominal pain and hematochezia. The mortality rate can reach 90.5% due to extensive intestinal necrosis, making surgical resection of the necrotic bowel tissue imperative.




