| disease | Pelvic Congestion Syndrome |
The most prominent symptoms of this syndrome include lower abdominal pain, low back pain, sexual discomfort, extreme fatigue, static blood dysmenorrhea, and premenstrual breast pain. The pain often worsens several days before menstruation and eases on the first or second day of the period, though a few cases involve persistent pain. The pain also frequently intensifies after prolonged standing, running, jumping, sudden sitting, or sexual intercourse, and is generally worse in the afternoon than in the morning. Deep dyspareunia is a common yet often unspoken symptom. In addition to pain, other common symptoms include excessive leucorrhea, constipation, bladder pain, and emotional dysphoria. Most affected patients are married, with symptoms typically appearing shortly after childbirth or late abortion. The syndrome is most prevalent in women aged 25–40, rarely occurs before menopause, and has never been observed postmenopause. During gynecological examinations, tenderness may be noted in the cervix, posterior fornix, and uterine body, with thickened adnexal areas and tenderness in the parametrial tissues also commonly observed.
bubble_chart Etiology
Any factor that hinders or obstructs the outflow of pelvic venous blood from the pelvic cavity can lead to pelvic venous static blood. Compared to men, the female pelvic circulation differs significantly in terms of anatomy, hemodynamics, and mechanics, forming the basis for the susceptibility to pelvic static blood.
1. Anatomical Factors
The characteristics of female pelvic circulation primarily include an increased number of veins and structurally weak vessels.
Medium-sized pelvic veins, such as the uterine veins, vaginal veins, and ovarian veins, are typically accompanied by 2–3 veins alongside a single homonymous artery. The ovarian veins can even number up to 5–6, forming a plexus of tortuous veins that curve behind the sides of the uterine body, only coalescing into a single ovarian vein before crossing the pelvic brim. Numerous anastomoses exist among the uterine, fallopian tube, and ovarian veins. Within the mesosalpinx, anastomoses between uterine and ovarian veins form a circular venous loop, which further connects with the lateral ovarian venous plexus. The venous plexuses originating from the mucosal, muscular, and serosal layers of pelvic organs converge into two or more veins that drain into the larger internal iliac veins. The increased number of pelvic veins serves to accommodate the slow flow of pelvic venous blood.
Pelvic veins have thinner walls compared to veins in other parts of the body, lack an outer sheath composed of fascia, and are devoid of valves and elasticity. They traverse loose connective tissues in the pelvis, making them prone to dilation and the formation of numerous tortuous venous plexuses. Small and medium-sized pelvic veins only develop valves just before they join larger veins, and some multiparous women may even exhibit valvular insufficiency. These features render the venous system of pelvic organs akin to a swamp interconnected by waterways, capable of accommodating large volumes of rapidly inflowing arterial blood.Additionally, the venous plexuses of the bladder, reproductive organs, and rectum are interconnected. Due to the lack of valves, circulatory disturbances in any one of these systems can affect the other two. (Figures 3 and 4)
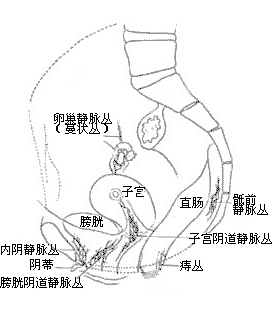
Figure 3: Lateral View of Pelvic Venous Plexuses (Showing the Locations of Six Venous Plexuses)
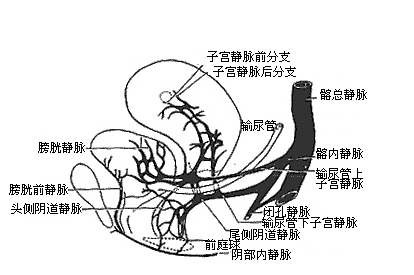
Figure 4: Relationship Between the Veins of the Bladder, Uterus, and Vagina and the Iliac Veins
(Illustrating the Drainage Tendency of Reproductive System Veins Along Two Main Trunks, Anterior and Posterior)
Against the backdrop of these anatomical features of pelvic veins, the influence of the following related factors can contribute to pelvic static blood syndrome, manifesting various clinical symptoms.
2. Constitutional Factors
Some patients, due to constitutional factors, exhibit significantly weak vascular walls, with fewer elastic fibers and reduced elasticity, predisposing them to venous stasis and varicosities. Even during their first pregnancy, without prolonged standing or sedentary work, they may develop varicose veins in the lower limbs and/or pelvis, along with pelvic static blood syndrome.
3. Mechanical FactorsVarious mechanical factors have been shown to affect the velocity of pelvic blood flow, thereby altering local vascular pressure, with veins being more susceptible to such influences.
(1) Posture: Individuals engaged in prolonged standing or sitting work are prone to sustained increases in pelvic venous pressure, leading to pelvic static blood syndrome. Such patients often report worsening abdominal pain, lumbago, increased leucorrhea, and heavier menstruation after prolonged standing or sitting, with symptoms alleviating after rest. Additionally, habitual supine sleepers may experience impaired pelvic venous outflow due to the gravitational pull of the uterine body and bladder distension displacing the uterus posteriorly. From a mechanical perspective, habitual supine sleeping positions place most pelvic veins below the level of the inferior vena cava, hindering venous outflow. In contrast, lateral or prone sleeping positions facilitate pelvic venous drainage.
(2) Uterine retroversion: Uterine retroversion accounts for 15-20% of gynecological diseases, and the proportion may be even higher among multiparous women. A hundred years ago, uterine retroversion was believed to be the cause of various pelvic symptoms, and uterine suspension procedures were frequently performed. By the beginning of this century, it gradually became recognized that the vast majority of mobile uterine retroversions are asymptomatic and require no treatment, with only a portion of retroverted uteruses having pathogenic effects. However, many doctors still believe that a small number of mobile uterine retroversions can indeed cause pelvic pain.
When the uterus is retroverted, the blood vessels of the ovarian plexus descend with the uterine body and bend on either side of the sacral concavity, increasing venous pressure and impairing venous return, leading to a state of static blood in the veins. If combined with a habit of sleeping in the supine position, this can eventually result in pelvic static blood syndrome.
(3) Early marriage, early childbirth, and frequent pregnancies: During pregnancy, the influence of large amounts of androgens and progestogens, along with the pressure exerted by the enlarged uterus on the surrounding veins, can cause dilation of the periuterine veins.(4) Constipation: Constipation affects the venous return of the rectum, and the veins of the rectum, uterus, and vagina are interconnected. Congestion of the hemorrhoidal plexus inevitably leads to congestion of the uterine and vaginal plexuses, so habitual constipation can easily contribute to pelvic static blood.
(5) Laceration of the broad ligament
A tear in the broad ligament membrane weakens its structure, reducing elasticity. Veins lacking an inherent vascular sheath lose support, leading to varicose veins and causing the uterus to tilt backward.
(6) Tubal ligation: With the widespread implementation of family planning, tubal ligation has become one of the most commonly performed procedures. In recent years, many journals have reported complications following ligation, such as lower abdominal pain, menstrual disorders, and secondary dysmenorrhea.
Tubal ligation is a minor procedure, and theoretically, it should not cause the aforementioned complications. However, in reality, some women do experience distressing and challenging complications post-ligation.
4. Autonomic nervous system dysfunction
Despite the various causes and anatomical pathologies mentioned above, many gynecologists believe that certain symptoms of pelvic static blood syndrome—such as depression, melancholy, dysphoria, fatigue, chronic pain, lumbago, and sexual discomfort—are largely related to the patient's mental state. These symptoms may result from autonomic nervous system dysfunction.
5. Other factors
Clinically, some patients with uterine fibroids, chronic pelvic inflammation (especially those with tubo-ovarian cysts), lactational amenorrhea, or moderate to severe (grade III) cervical erosion exhibit pelvic venous static blood during pelvic venography. Similarly, long-term depression, chronic illness, insomnia, and hormonal fluctuations during the premenstrual phase can also present symptoms resembling pelvic static blood syndrome. In the former cases, pelvic venous static blood may be considered a secondary change, while in the latter, it may act as an aggravating factor for pelvic static blood syndrome.
bubble_chart Pathological Changes
Gross pathological findings: The vulvar veins are engorged and varicose, the vaginal mucosa appears purplish-blue, the cervix is hypertrophic and edematous, and the cervical canal mucosa often exhibits everted erosion with surrounding mucosa showing purplish-blue discoloration. Occasionally, engorged small veins can be observed on the posterior lip of the cervix, and cervical secretions are abundant. Surgical observations reveal that in the majority of patients, the uterus is retroverted into the sacral concavity, with its surface displaying a purplish-blue static blood appearance or yellowish-brown static blood spots, along with subserosal edema. Engorged and varicose uterine veins are visible. The bilateral ovarian venous plexuses resemble a cluster of earthworm-like curves on either side of the retroverted uterine body, sometimes more pronounced on one side than the other, and occasionally abnormally enlarged like varicose veins. The veins within the mesosalpinx are also significantly thickened and engorged compared to normal, with diameters reaching 0.8–1.0 cm, some appearing varicose. When the uterus is repositioned to an anteverted position, peritoneal lacerations may be observed in the recesses of the posterior leaves of the broad ligaments. A few lacerations resemble widened eye fissures, extending inward toward the uterosacral ligaments as if absent. Some lacerations are smaller, while others exhibit thin posterior peritoneal leaves, through which engorged and varicose uterine veins bulge. Typically, within no more than 10 minutes, the anteverted uterus transitions from purplish-blue back to a normal pale red color. Microscopically, the uterine endometrial stroma shows edema, with veins engorged and dilated. The ovaries are generally enlarged, cystic, and edematous. The mammary glands exhibit edema and congestion, leading to breast distension and pain. In cases with broad ligament lacerations and third-degree uterine retroversion, the rectouterine pouch may contain 30–80 ml of pale blue serous fluid.
bubble_chart Clinical Manifestations
The main manifestations of pelvic static blood syndrome include a wide range of chronic pain, extreme fatigue, and certain neurasthenic symptoms. Among these, chronic lower abdominal pain, low back pain (lumbago), dyspareunia, extreme fatigue, excessive leucorrhea, and dysmenorrhea are the most common, with over 90% of patients experiencing these symptoms to varying degrees. Chronic pain refers to various forms of pain lasting more than six months, occurring no less than five days a week, with daily pain duration of at least four hours. Additionally, patients often experience hypermenorrhea, premenstrual breast distending pain, premenstrual defecation pain, bladder irritation symptoms, vaginal dragging pain, and anal dragging pain. These symptoms typically worsen in the afternoon, evening, or after standing, and become more severe after intercourse or just before menstruation.
1. Lower abdominal pain
Most cases involve chronic diffuse pain above the pubic symphysis or bilateral lower abdominal pain, often more severe on one side, sometimes extending to the same side of the lower limb, particularly the groin or hip, with soreness and weakness. This pain usually begins during the intermediate stage of menstruation [second stage]. A few patients may occasionally experience acute episodic abdominal pain, which can be misdiagnosed as acute appendicitis, follicular rupture, or ectopic pregnancy rupture.
2. Low back pain (lumbago)
Patients describe the pain as occurring at the sacro-gluteal region, sometimes in the lower half of the sacrum, often accompanied by lower abdominal pain. Symptoms worsen premenstrually, after prolonged standing, or following intercourse.
3. Dysmenorrhea
Over half of the patients experience this symptom. It typically begins several days before menstruation as lower abdominal pain, lumbosacral pain, or pelvic dragging and distending pain, sometimes progressing to cramping pain. The pain peaks on the day before or the first day of menstruation and significantly improves after the second day.
4. Dyspareunia
When questioned, patients often report varying degrees of pain during intercourse, mostly deep dyspareunia, sometimes unbearable. The pain not only occurs during intercourse but also worsens the next day, with increased lower abdominal pain, lumbago, and excessive leucorrhea, leading to aversion to sexual activity.
5. Extreme fatigue
Patients often feel extremely fatigued throughout the day, almost unable to complete their work (including household chores).
6. Excessive leucorrhea
Over half of the patients experience excessive leucorrhea, usually clear mucus without signs of infection.
7. Menstrual changes
Some patients exhibit hypermenorrhea, often misdiagnosed as uterine fibroids or uterine hypertrophy due to an enlarged uterus. Others may have reduced menstrual flow but with noticeable premenstrual breast pain.
8. Static blood-related breast pain
Over 70% of patients experience static blood-related breast pain and swelling. Patients may palpate breast nodules with tenderness, usually appearing after the intermediate stage of menstruation [second stage] alongside other symptoms, peaking the day before or the first day of menstruation. Symptoms may lessen or disappear after menstruation. Some patients report breast pain as more severe than pelvic pain, becoming their primary complaint.
9. Vulvar and vaginal swelling, dragging pain
Patients with pelvic static blood syndrome often experience swelling and dragging pain in the vulva and vagina, or vulvar burning and cutaneous pruritus. The vulva may appear pigmented, with swollen or hypertrophic labia, sometimes accompanied by venous engorgement, distension, or varicosities.
10. Bladder and urethral symptoms
Over one-third of patients experience frequent urination and dysuria premenstrually, often mistaken for urinary tract infections, though urinalysis is normal. Cystoscopy in severe cases may reveal venous engorgement, congestion, and edema in the bladder trigone. Rarely, rupture of static blood veins may lead to hematuria.
11. Rectal dragging pain
Some patients experience varying degrees of rectal dragging pain, rectal pain, or pain during defecation, more pronounced premenstrually, especially in cases of third-degree retroverted uterus.
12. Autonomic nervous system symptoms
The vast majority of patients with pelvic static blood syndrome are accompanied by certain symptoms of the autonomic nervous system. Although their manifestations and severity may vary, they generally fall into the following aspects:
(1) General neurological symptoms: dysphoria, irritability, proneness to anger and crying, low mood or melancholy, frequent night dreams, often severe daytime fatigue and a sense of mental and physical incapacity. Headache is common, mostly occipital pain rather than the typical premenstrual headache type.
(2) Cardiovascular aspects: May include palpitations and a sense of chest tightness or discomfort in the precordial area.
(3) Respiratory system: A sensation of shortness of breath, often requiring forceful deep inhalation.
(4) Digestive system: Symptoms include belching, abdominal distension and fullness, and a feeling of incomplete gas expulsion. Subjective poor appetite and indigestion are reported, though actual food intake is not reduced, and no weight loss occurs.
(5) Others: Unexplainable aches and discomforts throughout the body, such as shoulder arthralgia, hip arthralgia, finger tightness, and in many cases, a sensation of eyeball pressure.
13. Signs
Disproportionate to the severity of the subjective symptoms described above. The only finding upon abdominal examination is tenderness, usually located in the suprapubic region or as deep tenderness in the lower abdomen on both sides. Generally, it is not significant, and there is often no consistent, particularly prominent tender point. Even at the site where the patient feels the most pain, there is no abdominal rigidity or rebound tenderness. During gynecological bimanual examination, the uterus is often found to be retroverted, slightly enlarged or normal. The uterine cervix is hypertrophied, appearing purplish-blue, mostly smooth, though some cases show erosion.
Some patients report feeling hard nodules in their breasts, but examination reveals only diffusely swollen mammary tissue beneath the nipples, often accompanied by varying degrees of tenderness.
As mentioned earlier, the symptoms of pelvic static blood syndrome involve a wide range, and the signs often overlap with certain other pathological conditions, making clinical diagnosis difficult. However, by taking a detailed medical history, paying attention to the differences in symptoms and signs, ruling out other related diseases, and appropriately applying pelvic venous calm pulse angiography and laparoscopy, a relatively reliable diagnosis can be made. The following points may serve as references:
1. The patients are mostly women of childbearing age who have had two or more deliveries or late abortions. Shortly after a delivery or late abortion, they develop the aforementioned chronic pelvic pain, low back pain, dyspareunia, dysmenorrhea, and other symptoms, but there is no history of postpartum or post-late abortion infection.
2. There is a discrepancy between subjective symptoms and objective findings. Patients report numerous and severe symptoms, but gynecological examinations reveal only cervical hypertrophy, cyanosis, and sometimes erosion, with the uterus retroverted into the sacral concavity. However, sudden manipulation of the cervix or pressure on the posterior fornix can cause significant pelvic and lumbosacral pain. The adnexal areas show marked tenderness and a sense of fullness. Upon gentle and prolonged palpation, a soft, spongy sensation is noted, without the thickening or hard cord-like structures typical of chronic adnexitis. There is also no abdominal muscle tension or rebound tenderness. Attempts to manually reposition the retroverted uterus to an anteverted position cause unbearable pain for the patient.
3. The condition is often accompanied by premenstrual breast swelling and tenderness, as well as certain neurasthenic symptoms.
4. Although patients may have been diagnosed with "chronic adnexitis" or "chronic pelvic inflammation," these conditions rarely hinder pregnancy. Some patients even become pregnant again soon after a late abortion, despite being diagnosed with chronic adnexitis and before their symptoms have resolved.
5. Previous treatments for chronic pelvic conditions have shown little to no effect. Patients feel they have a severe gynecological disorder that persists despite long-term treatment, leading gynecologists to consider it a refractory condition.
6. For patients with the aforementioned symptoms, gynecological examinations and other auxiliary diagnostic methods, such as hysterosalpingography, can be used to rule out organic sexually transmitted diseases in the pelvis. If pelvic venous static blood syndrome is clinically suspected, pelvic venography can assist in diagnosis. Specific procedures are detailed later.
7. Pelvic Venography Pelvic venography involves injecting a contrast agent into the myometrium at the base of the uterine cavity to visualize the uterine veins, ovarian veins, and parts of the vaginal and internal iliac veins. Continuous imaging at set intervals helps assess the time it takes for pelvic blood (primarily uterine and ovarian venous blood) to exit the pelvis, serving as an auxiliary diagnostic method for static blood syndrome. Under normal pelvic venous circulation, the contrast agent typically exits the pelvis completely within 20 seconds. In cases of pelvic static blood syndrome, venous return is significantly slower, and the contrast agent takes more than 20 seconds to leave the pelvis.
bubble_chart Treatment Measures
Before treatment, it is essential to first clarify the disease-causing factors in patients with pelvic static blood syndrome and carefully assess the severity of the condition.
I. Treatment for Mild Cases
Many patients experience symptoms shortly after childbirth or late abortion, or occasionally within 1–2 menstrual cycles, often not requiring medication. Health guidance tailored to the disease cause should be provided to ensure the patient fully understands the formation, prevention, and management of the condition. For example, during midday and evening rest periods, changing from the habitual supine position to a lateral prone position (Figure 1), correcting constipation, moderating sexual activity, and engaging in appropriate physical exercise to enhance pelvic muscle tone and improve pelvic blood circulation generally yield good results. For those with cervical erosion, timely treatment of cervical erosion leads to more satisfactory outcomes.
II. Treatment for Severe Cases
Some patients have endured years of suffering with no relief despite multiple medical consultations. Thus, it is crucial to ensure the patient fully understands the condition, builds confidence in overcoming it, and actively cooperates with treatment. At midday and evening, patients should consistently perform a knee-chest position for over 10 minutes, followed by resting in a lateral prone position to observe the effects. This approach often significantly alleviates or mitigates severe pelvic pain. Based on the principle that "free flow prevents pain," treatments such as invigorating blood and resolving stasis (e.g., Salvia, Carthamus, Sichuan Lovage Rhizome, Chinese Angelica, Peach Kernel, Typha, Trogopterus Dung, etc.) and tuina therapy can be effective. For patients with severe breast tumors or hypermenorrhea, starting low-dose methyltestosterone before symptom onset may also help.
If the lateral prone position therapy is effective but not sustainable, surgical intervention may be considered. The choice of surgical method should account for the patient’s age, fertility needs, symptom duration, and whether organic sexually transmitted disease changes are present. The following methods may be selected as appropriate:
(1) Round Ligament Suspension: Surgically maintaining a retroverted uterus in an anteverted position often reduces the size of an enlarged uterine body and cervix, significantly alleviating or eliminating pelvic pain. The best method involves passing the round ligament around the lateral edge of the rectus abdominis near the internal inguinal ring, securing it between the rectus abdominis muscle and its anterior sheath. This minimizes the gap between the tightened ligament and the parietal peritoneum, unlike the modified Gilliams method, which pulls the ligament outside the anterior sheath and sutures it externally, often causing postoperative abdominal wall pain. Pain is clearly localized where the ligament pierces the fascia, and palpation of this area can trigger discomfort and reveal the displaced ligament.
(2) Broad Ligament Laceration Repair: Suitable for young patients with severe pelvic static blood syndrome due to broad ligament lacerations who no longer wish to conceive. Preoperatively, the presence of such lacerations cannot be confirmed. However, for patients with a severely retroverted uterus (grade III), intense pelvic pain, and no desire for further fertility but unwilling to undergo hysterectomy, round ligament suspension and tubal ligation may be planned. During laparotomy, if broad ligament lacerations (often on the posterior leaf) are discovered, they are repaired first, followed by tubal ligation and round ligament suspension. Thus, this typically involves a combination of procedures (Figures 1 and 2).

Figure 1: Lateral Prone Position
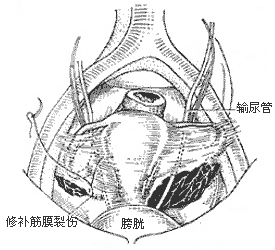
(1) Anterior Broad Ligament Fascial Laceration and Repair

(2) Broad Ligament Laceration and Repair
Figure 2: Broad Ligament Laceration and Repair
Since Allen and Master first reported it in 1995, over 30 years of surgical practice and exploration by more than 10 scholars involving over 300 cases have proven that this set of procedures can achieve good therapeutic outcomes for the vast majority of patients. All patients' uteruses returned to normal position and size, with symptoms and signs almost completely disappearing.
Although this surgery is simple, it requires meticulous operation and is performed abdominally. There is no need to trim the edges of the laceration. Starting from the connection between the cervix and the paracervical abdominal membrane, interrupted sutures with No. 1 silk thread are gradually placed outward. As long as the first stitch ensures the edge of the laceration is secured, the boundaries of the laceration become clearly visible during subsequent suturing. Care must be taken to avoid the course of the ureter, ensuring it is not mistakenly sutured, not even its sheath membrane. Once the laceration is sutured, the bulging varicose veins under the abdominal membrane also disappear, the cardinal ligament becomes firm and strong, and the relaxed sacral ligament is shortened. This way, the uterus can maintain an anteverted position. Then, the fallopian tubes are ligated, and finally, a round ligament suspension is performed.
(3) Total abdominal hysterectomy with adnexectomy: For women over 40 years old with severe conditions, especially those complicated by hypermenorrhea or approaching menopause, total abdominal hysterectomy with adnexectomy yields better results. Abdominal surgery has more advantages over vaginal surgery, as it allows for the removal of more varicose pelvic veins, particularly uterine and ovarian veins (one of the main objectives of the surgery), and facilitates the repair of injuries to the broad ligament and sacral ligament, ensuring better fixation of the vaginal stump. Additionally, abdominal surgery is less likely to injure the increased varicose veins within the broad ligament, resulting in less bleeding. When one ovary needs to be preserved, abdominal surgery allows for more appropriate fixation of the ovary.
(4) Other procedures: Simple round ligament shortening, unilateral salpingo-oophorectomy, or presacral neurectomy generally yield unsatisfactory results. Ligation of varicose veins is particularly inadvisable.
Implement the principle of "prevention first," and take preventive measures against the causes of pelvic static blood disease to avoid or reduce its occurrence.
Strengthen family planning education to prevent early marriage, early childbirth, frequent intercourse, and closely spaced births. Advocate for having no more than two children, with at least a 3-5 year interval between births, allowing the reproductive organs to fully recover not only anatomically and physiologically but also in terms of vascular function. Promote scientific contraceptive methods and avoid the use of withdrawal as a contraceptive. Abstinence is also not recommended.
Emphasize physical exercise to enhance constitution and improve overall health, which is particularly important for individuals with weaker constitutions.
Strengthen postpartum hygiene education and promote postpartum exercises, which greatly benefit the recovery of reproductive organs and their supporting tissues. Avoid habitual supine positions during rest or sleep, and advocate for alternating side-lying positions to help prevent the formation of a retroverted uterus. Preventing severe postpartum constipation and urinary retention aids in the recovery of reproductive organs and pelvic venous return.
Pay attention to balancing work and rest to avoid excessive fatigue. For those engaged in prolonged standing or sitting work, workplace exercises and appropriate activities should be introduced whenever possible. Additionally, whether one can fall asleep or not, resting in bed for a period at noon can alleviate morning fatigue. However, it is worth noting that different postures during bed rest or sleep have varying effects on relieving fatigue and improving pelvic blood circulation. From a biomechanical perspective, in the supine position, most pelvic veins are positioned lower than the inferior vena cava, resulting in significantly higher venous pressure compared to side-lying or prone positions, though it is still lower than when standing or sitting. Ancient health practices emphasized the adage: "Stand like a pine, sit like a bell, and lie like a bow." Therefore, adopting the habit of a flexed-leg side-lying position can be highly effective in preventing and even treating mild cases of pelvic static blood disease.




