| disease | Congenital Mitral Valve Malformation |
Congenital mitral valve malformations can lead to mitral stenosis and/or insufficiency. Fisher reported two cases of congenital mitral valve malformation as early as 1902, and since the 1960s, reported cases have increasingly multiplied. Congenital mitral valve malformations are often associated with various other congenital cardiovascular anomalies, such as atrioventricular canal defects, transposition of the great arteries, single ventricle, atrial septal defects, ventricular septal defects, left ventricular outflow tract or aortic outlet stenosis, aortic coarctation, and tetralogy of Fallot. Isolated congenital mitral valve malformations are rare, accounting for only 0.6% of autopsy cases of congenital cardiovascular diseases and 0.21–0.42% of clinical cases. The mitral valve consists of the annulus, leaflets, chordae tendineae, papillary muscles, and the left atrial wall. Developmental abnormalities in any one or several of these components can result in congenital mitral valve malformations. In most cases, multiple components are developmentally abnormal, leading to multiple sites of stenosis in the left atrioventricular blood flow pathway.
bubble_chart Pathological Changes
(1) The following abnormalities are commonly seen in mitral stenosis (Figure 2):
(1) Excessive leaflet tissue | (2) Parachute-type stenosis | (3) Fusion of papillary muscles and commissures, with absence of chordae tendineae |
(4) Funnel-shaped stenosis due to fistula disease | (5) Supravalvular ring | (6) Chordae tendineae attached to a single papillary muscle, dividing the valve orifice into multiple small orifices |
Figure 2: Pathological changes in congenital mitral stenosis
1. Hypoplasia of the mitral annulus without other valve membrane abnormalities. The annulus is 20–50% smaller than normal, resulting in varying degrees of mitral stenosis.
2. Absence of chordae tendineae, with two thickened papillary muscles directly fused to the valve commissures. The valve membrane forms a funnel shape due to fistula disease, restricting leaflet movement and causing severe stenosis at the orifice between the papillary muscles.
3. All chordae tendineae are attached to the apex of a single, large papillary muscle, with the interchordal spaces occluded by excessive leaflet tissue. The mitral valve assumes a parachute shape, a relatively common deformity.
4. Two normally developed papillary muscles, but the interchordal spaces are occluded by abnormal leaflet tissue. Sometimes, excess leaflet tissue forms a bridging structure between the anterior and posterior leaflets, dividing the mitral valve into two orifices.5. Absence of normal papillary muscles, replaced by numerous fine fibrous or muscular bundles on the posterior ventricular wall. The anterior leaflet chordae traverse the valve orifice and attach to abnormal papillary-like tissue on the posterior ventricular wall, dividing the orifice into multiple small orifices.
6. Supravalvular ring: A ring of fibrous tissue appears below the left atrial wall near the mitral annulus. A narrow fibrous ring may not affect mitral orifice flow, whereas a wide supravalvular fibrous ring can partially cover the mitral orifice, causing stenosis.
(2) The following deformities commonly cause mitral regurgitation (Figure 3):
| (1) Leaflet cleft | (2) Leaflet dysplasia forming small holes | ||
| (3) Leaflet prolapse | (4) Overly large annulus | (5) Parachute valve membrane | |
Figure 3: Pathological changes in congenital mitral regurgitation
1. Leaflet cleft: Clefts in the anterior leaflet are most common. The free edge of the cleft is often attached to abnormal chordae tendineae. In a few cases, the cleft is located in the posterior leaflet. Sometimes, the mitral valve has three clefts, forming three leaflets with enlarged commissures, leading to regurgitation.
2. Partial leaflet dysplasia or formation of small holes. This deformity is more common in the posterior leaflet.
3. Leaflet prolapse: An excessively long or large anterior leaflet, missing chordae tendineae, overly long chordae tendineae, or overly long papillary muscles can all cause the free edge of the leaflet (commonly the anterior leaflet) to prolapse into the left atrium during cardiac contraction, resulting in regurgitation.
4. Annular enlargement, causing the anteroposterior diameter of the mitral valve orifice to exceed the transverse diameter, preventing the two leaflets from coapting during cardiac contraction.
5. Restricted leaflet mobility due to overly short chordae tendineae or abnormal papillary muscles, causing the mitral valve to take on a parachute or funnel shape, as well as missing papillary muscles. Both the anterior and posterior leaflets may attach to the left ventricular posterior wall via abnormal chordae tendineae, or the anterior papillary muscle may be underdeveloped, leading to corresponding underdevelopment of the anterior leaflet portion and the anterolateral commissure, forming commissural clefts, all of which can result in regurgitation.
The clinical manifestations of congenital mitral valve malformation are similar to those of acquired mitral valve disease, but symptoms appear earlier, and there is no history of wind-dampness Rebing. Approximately 30% of patients develop symptoms within 1 month after birth, and 75% within 1 year after birth. Common symptoms include shortness of breath, orthopnea, pulmonary edema, and recurrent pulmonary infections. In severe cases, due to complications such as pulmonary hypertension, congestive heart failure and cyanosis may occur.
Physical examination: Poor physical growth and development, and easy fatigue. The typical sign of mitral stenosis is a diastolic rumbling murmur at the apex, which may be accompanied by a thrill, a loud first heart sound, and an opening snap. If the valve leaflets are restricted in movement, these signs may not be obvious. In cases complicated by pulmonary hypertension, the second heart sound at the pulmonary valve area is accentuated and split. Moist rales may be heard in patients with pulmonary infections. In cases of mitral regurgitation, a forceful heaving apical impulse may be visible and palpable, and a pansystolic murmur radiating to the left axilla can often be heard at the apex, along with a third heart sound. In cases of pulmonary hypertension, the second heart sound at the pulmonary valve area is accentuated and split.
bubble_chart Auxiliary Examination
Chest X-ray examination: The cardiac shadow is enlarged, with significant abnormal enlargement of the left atrium. The pulmonary stirred pulse cone is prominent, and pulmonary congestion leads to thickening of the pulmonary vascular markings. In severe cases, signs of pulmonary edema may be present. Electrocardiogram examination: The typical signs include widened P waves with notches. Biphasic P waves in the right chest leads indicate left atrial hypertrophy. Cases of mitral stenosis show right ventricular hypertrophy and right axis deviation. Cases of mitral regurgitation show left ventricular hypertrophy and strain or hypertrophy of both ventricles. Atrial fibrillation is rare. Cardiac catheterization and selective left heart angiography: These examinations can reveal the location, morphology, and severity of the lesion, determine pulmonary circulation pressure and pulmonary vascular resistance, and assess any coexisting congenital cardiovascular diseases.
bubble_chart Treatment Measures
Congenital mitral valve malformation cases, such as mitral valve lesions with mild impact on cardiac function and no accompanying other cardiovascular malformations, may present clinical symptoms later in life and survive into adulthood. If clinical symptoms appear within the first year after birth, the condition often progresses rapidly and worsens continuously, with approximately half of the cases dying between 6 months and 1 year of age, and very few surviving beyond 10 years. Therefore, surgical treatment should be pursued once a definitive diagnosis is made.
Treatment: Starky initiated surgical treatment for congenital mitral valve malformation in 1959, followed by Creech in 1962. Due to the complexity and morphological diversity of congenital mitral valve malformations, as well as the rarity of the condition, the number of surgical cases is limited, and accumulated experience is scarce. There are still many differing opinions regarding surgical methods. The age for surgery should consider factors such as physical growth and development, physical activity capacity, and the presence of complications like pulmonary hypertension. The surgical mortality rate for infant cases is as high as 50%. Collagen tissue is not yet mature within the first 3 months after birth, and the valve tissue is very fragile, making it prone to damage during surgery. If the condition permits, surgery should ideally be delayed until 2–3 years of age. For cases complicated by grade III pulmonary hypertension, surgery should be performed before 1.5 years of age. Mitral valve repair is the preferred surgical method. In pediatric cases, the use of mechanical valves is prone to postoperative thromboembolism, requiring long-term anticoagulation therapy. Although bioprosthetic valves do not require long-term anticoagulation and have better hemodynamic performance, the incidence of valve calcification is very high. Therefore, mitral valve replacement should be avoided as much as possible in pediatric patients. Cases requiring valve replacement due to the severity of the lesions account for about 10–15%.
Surgical procedure: A median sternotomy is performed, and cannulas are inserted into the superior and inferior vena cava via a right atrial incision. An ascending aortic cannula is placed near the opening of the innominate artery. Cardiopulmonary bypass is established, and the body temperature is lowered to around 25°C, with myocardial protection achieved by local cooling using cold cardioplegic solution. After aortic cross-clamping, the left atrium is incised along the interatrial groove to assess the mitral valve lesions, with particular attention to leaflet mobility, coaptation, and the morphology of the annulus, chordae tendineae, and papillary muscles. Intraoperative pressurization of the left ventricular cavity with saline can help evaluate mitral valve function. If the leaflet closure line is parallel to the base of the posterior-medial leaflet at the annulus and there is sufficient coaptation between the two leaflets, the valve malformation can be considered satisfactorily corrected.
Repair of congenital mitral stenosis: The following corrective procedures are performed based on the specific valve lesions (Figure 1).
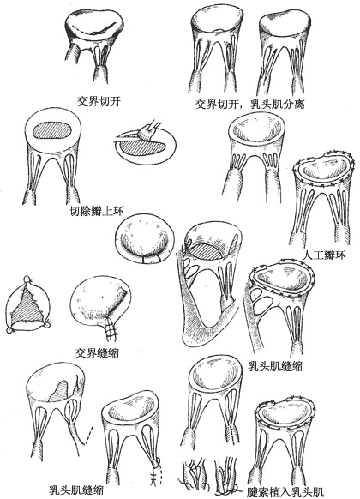
Figure 1: Repair of congenital mitral valve malformations
Leaflets: Perforated leaflets can be patched with pericardium, and cleft leaflets can be sutured intermittently. Fused commissures can be treated with commissurotomy.
Chordae tendineae: Overly long chordae can be implanted into the papillary muscle. For partial absence of chordae, the unsupported segment of the leaflet can be excised, and the leaflet and annulus can be sutured. If the interchordal spaces are obscured by leaflet tissue or the chordae are too thick, resulting in narrow spaces, chordal fenestration can be performed. Alternatively, the free edge of the leaflet lacking chordae can be sutured and fixed to adjacent chordae.
Papillary muscles: For overly long papillary muscles or a single large papillary muscle, partial incision or fenestration of the papillary muscle can be performed.
Annulus: An overly large annulus can be treated with annuloplasty using sutures or an artificial annuloplasty ring. For a supramitral ring, the fibrous ring is excised along the left atrial wall, taking care not to injure the anterior leaflet.
Valve replacement: After excision of the mitral valve, the annulus is gently dilated using a uterine cervical dilator. Only the struts of the prosthetic valve are placed into the valve orifice, while the sewing ring is sutured to the left atrial wall. This allows the use of a larger-sized prosthetic valve.
Treatment outcome: In cases of mitral stenosis, the surgical mortality rate for valve membrane repair and plasty is 25-40%. For patients with mitral insufficiency, the surgical mortality rate is only 3-10%. The surgical mortality rate for valve membrane replacement in mitral stenosis cases is 20-60%, and for mitral insufficiency, it is 30%. Although more than half of the surviving cases still have residual diastolic pressure gradient between the left atrium and left ventricle or systolic murmurs can still be heard in the apical region, nearly 90% of the cases recover to functional class I.






