| disease | Acute Renal Failure (Surgical) |
Acute renal failure is a general term for acute kidney parenchymal damage secondary to disease causes such as shock, trauma, severe infection, hemolysis, and poisoning, constituting a syndrome. Its main pathological change is renal tubular necrosis, clinically presenting with oliguria or anuria, accompanied by severe water-electrolyte imbalances, metabolic disturbances, and uremia. In recent years, another type of acute renal failure with normal or increased urine output has been observed, characterized by normal or higher urine volume but progressively worsening azotemia leading to uremia, known as non-oliguric acute renal failure.
bubble_chart Etiology
Acute renal failure has many disease causes, and based on these disease causes, acute renal failure can be classified into the following five clinical types.
(1) Shock-type acute renal failure: Shock caused by various disease causes can lead to acute renal failure. Common disease causes include hemorrhage, water-electrolyte imbalance, and cardiogenic circulatory failure.
(2) Infection-type acute renal failure: Infections caused by bacteria, viruses, or fungi can all complicate into acute renal failure. Viral infections that frequently lead to acute renal failure include viral pneumonia, encephalitis, hepatitis, and epidemic hemorrhagic fever. Bacterial infections, especially those caused by Gram-negative bacteria, are prone to induce acute kidney failure.
(3) Crush-type acute renal failure: This is caused by severe crush injuries. Its disease causes and clinical course are extremely complex. It is a common and clinically significant type.
(4) Hemolytic-type acute renal failure: Incompatible blood transfusions, massive transfusions of outdated blood, and mechanical hemolysis can all complicate into acute renal failure. The main pathogenesis is disseminated intravascular coagulation.
(5) Toxic-type acute renal failure: There are many types of toxins that can cause acute kidney failure, which can be summarized into the following four categories: ① Heavy metal compounds such as mercury. ② Organic compounds such as DDT and dichlorvos. ③ Biological toxins such as snake venom and poisonous mushrooms. ④ Nephrotoxic drugs such as nephrotoxic antibiotics.
bubble_chart PathogenesisIn acute renal failure, renal microcirculatory disturbance, renal ischemia, and disseminated intravascular coagulation are the three central links in the pathogenesis. The details are described as follows:
(I) Renal Microcirculatory Disturbance
1. The Role of Catecholamines in Pathogenesis: During the treatment of epidemic cerebrospinal meningitis, bacterial pneumonia, toxic dysentery, and drug-induced anaphylactic shock, it has been observed that microvascular spasms in the fundus and elevated blood catecholamine levels occur. Therefore, it is certain that microcirculatory disturbances leading to functional oliguria are mediated by catecholamines.
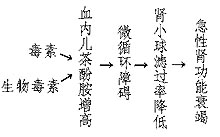
Figure 1: The Role of Catecholamines in the Pathogenesis of Acute Kidney Failure
2. The Role of the Renin-Angiotensin System in Pathogenesis: Renal ischemia or toxins can cause renal tubular injury, reducing sodium reabsorption in the proximal tubules and increasing sodium concentration at the macula densa. This triggers the release of renin and an increase in angiotensin II, leading to the constriction of preglomerular arterioles, reduced renal blood flow, and decreased glomerular filtration rate, ultimately resulting in acute renal failure.
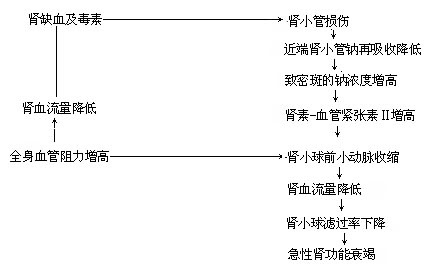
Figure 2: The Role of the Renin-Angiotensin System in the Pathogenesis of Acute Kidney Failure
(II) Renal Ischemia
1. Renal Ischemia Caused by Renal Vasoconstriction: Under normal circumstances, the kidneys receive a rich blood supply, accounting for 20–25% of cardiac output. In shock caused by various factors, the body constricts peripheral arterioles, including renal arterioles, to ensure blood supply to vital organs such as the heart and brain. Consequently, renal blood flow decreases, leading to renal ischemia.
2. Ischemia Due to Renal Shunt Circulation: The renal blood circulation follows two pathways. The first involves blood flowing through renal arterioles, arcuate arterioles, interlobular arterioles, afferent and efferent arterioles, and then converging into peritubular capillaries before entering the renal venous system. The second is a shunt circulation where blood flows through interlobular arterioles directly into the vasa recta and then into the venous system, bypassing the glomerular arterioles. Normally, 90% of blood follows the first pathway, while only 10% takes the shunt route. Under severe stress such as trauma, shock, or infection, renal vasoconstriction occurs as a protective mechanism, causing a paradoxical shift where over 90% of blood flows through the shunt circulation. This drastically reduces blood supply to the renal cortex and tubules, leading to acute renal failure.
In shock caused by various factors, blood pressure drops, tissue blood flow decreases, and capillary blood flow slows, resulting in cellular hypoxia. This leads to the release of thromboplastin and accumulation of lactic acid, increasing blood hypercoagulability. Additionally, vascular endothelial injury caused by trauma, bacterial toxins, acidosis, and hypoxia promotes platelet and red blood cell aggregation and destruction, releasing procoagulant substances that activate the coagulation system. This results in microvascular clotting and thrombosis. The coagulation and thrombosis within renal microvessels inevitably worsen renal ischemia, ultimately leading to acute renal failure.
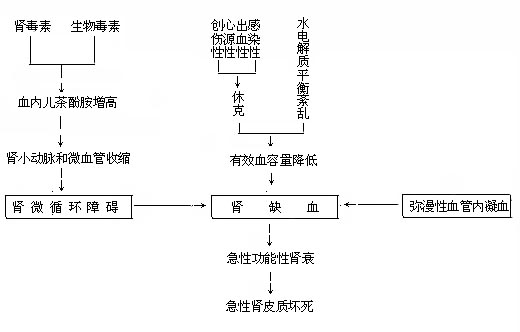
Figure 3: The Pathogenic Mechanism of Acute Renal Failure
bubble_chart Clinical Manifestations
The clinical course of acute renal failure is divided into four stages: the onset stage, the oliguric or anuric stage, the diuretic stage, and the stage of convalescence. In cases caused by poisoning, the onset stage may be absent.
The pathophysiological changes and clinical manifestations of each stage are described below:
(1) Onset stage: When the body experiences disease causes such as shock, hypovolemia, and decreased blood pressure, renal vasoconstriction occurs, leading to reduced renal blood flow and decreased glomerular filtration rate, resulting in decreased urine output. Additionally, the body's response increases the secretion of antidiuretic hormone, aldosterone, and adrenocorticotropic hormone, further reducing urine output, increasing specific gravity, and decreasing urinary sodium. This stage is primarily characterized by hypovolemia and renal vasospasm, with clinical manifestations limited to the signs of the primary disease and oliguria. This stage is crucial for preventing the progression of acute renal failure. If managed promptly and appropriately, it can avoid progression to the stage of organic renal failure.
(2) Oliguric or anuric stage: Persistent disease causes can lead to damage to the kidney parenchyma, mainly degeneration and necrosis of renal tubular epithelial cells, thereby entering the oliguric or anuric stage. A 24-hour urine output of less than 400 ml is termed oliguria, and less than 100 ml is termed anuria. The main clinical manifestations of this stage include:
(1) Oliguria or anuria: The mechanisms of oliguria include: ① Reduced renal blood flow and decreased glomerular filtration rate, leading to decreased urine formation. ② Renal interstitial edema and increased pressure further impair renal blood flow, resulting in oliguria. ③ Rupture of the basement membrane of renal tubular epithelial cells, allowing communication between the tubular lumen and renal interstitium, leading to urine reflux into the interstitium and back into the venous system. ④ Pigment casts obstructing renal tubules and hindering urine excretion. The decrease in urine output during the oliguric stage can occur suddenly or gradually. The oliguric stage typically lasts 7–14 days. The shorter the oliguric stage, the better the prognosis. In cases of non-oliguric acute renal failure, urine output does not decrease. During this stage, the urine is acidic, with a fixed specific gravity around 1.010, generally below 1.014. Patients with hemolytic or crush-type acute renal failure may exhibit hemoglobinuria or myoglobinuria. Urinalysis may show protein, and microscopy may reveal red blood cells, granular or red blood cell casts. Urinary sodium content is increased, while urea and creatinine concentrations are decreased.
(2) Water intoxication: In cases of reduced renal excretion and excessive endogenous water production due to heightened metabolism, excessive intake of fluids and sodium salts can lead to water intoxication. This is a severe complication of the oliguric stage, clinically manifested as generalized soft tissue edema, acute pulmonary edema, and cerebral edema. In early pulmonary edema, only rales at the lung bases and diminished breath sounds are present. In severe cases, the entire lung is filled with bubbling breath sounds, accompanied by dyspnea and cyanosis. Cerebral edema presents with headache, vomiting, confusion, and spasms. Water intoxication can, on one hand, exacerbate cardiac burden due to systemic water retention, leading to heart failure, and on the other hand, cause electrolyte disturbances, endangering the patient's life. Therefore, water intoxication is one of the primary causes of death in acute renal failure.
2. Electrolyte disturbances
(1) Hyperkalemia: A serum potassium level above 7 mEq/L in adults is termed hyperkalemia. It is the most severe complication of acute renal failure and one of the primary causes of death. The main causes of hyperkalemia are reduced excretion, increased endogenous production, and increased intake. During the oliguric stage, reduced urinary potassium excretion leads to potassium accumulation in the body. Tissue injury, infection, insufficient caloric intake leading to heightened cellular catabolism, metabolic acidosis, and hypoxia can all cause potassium to leak out of cells, increasing serum potassium levels. Further intake of potassium-rich foods or massive transfusion of stored blood can further elevate serum potassium.
The main manifestations of hyperkalemia are signs of the circulatory system, such as bradycardia, arrhythmia, and hypotension, which can lead to cardiac arrest in severe cases. Secondary symptoms include dysphoria, mental confusion, sluggish reactions, abnormal sensations in the hands and feet, muscle soreness, and limb numbness. Electrocardiogram (ECG) changes often appear before clinical symptoms become obvious, such as tall T waves, disappearance of P waves, widened QRS complexes, and even ventricular fibrillation or cardiac arrest.
The occurrence of hyperkalemia symptoms is related to the patient's blood sodium and calcium concentrations. Symptoms may not be obvious when blood sodium and calcium levels are normal, but are more likely to appear when these concentrations decrease. If acidosis is also present, the symptoms of hyperkalemia are more prone to occur.
(2) Hyponatremia: Hyponatremia in acute renal failure is often dilutional. The reasons include increased extracellular fluid diluting sodium, sodium ions being used to neutralize acidic substances and excreted in urine, or entering cells to exchange with potassium ions. True sodium-deficient hyponatremia only occurs if conditions like vomiting, diarrhea, or extensive burns existed before acute renal failure. Generally, grade II hyponatremia may be asymptomatic or manifest as fatigue, sunken eyes, dizziness, or apathy. In severe cases, cerebral edema may occur, leading to hypotonic unconsciousness.
(3) Hyperphosphatemia: When renal function fails, phosphate excretion is impaired, resulting in hyperphosphatemia. It does not cause symptoms itself but can further lower blood calcium levels.
(4) Hypocalcemia: Due to impaired renal phosphate excretion, phosphate is instead excreted through the intestines, binding with calcium to form non-absorbable salts, leading to hypocalcemia. Since acidosis increases calcium ionization, clinical symptoms may not appear. However, once acidosis is corrected, hypocalcemic spasms may occur.
(5) Hypermagnesemia: Normally, magnesium is excreted by the kidneys, so renal failure can cause hypermagnesemia. Normal blood magnesium is 1.5–2.5 mEq/L. Symptoms such as loss of deep tendon reflexes, tachycardia, various heart blocks, hypotension, and muscle weakness appear when levels exceed 6 mEq/L. Severe cases may lead to drowsiness and unconsciousness.
3. Metabolic acidosis: In acute renal failure, acid retention depletes alkali reserves, and impaired renal tubular hydrogen and ammonia secretion prevents sodium and alkaline phosphate reabsorption, leading to metabolic acidosis. This acidosis is often progressive and difficult to correct, clinically manifesting as weakness, drowsiness, unconsciousness, weakened heart contractions, hypotension, and exacerbation of hyperkalemia.
4. Azotemia: In acute renal failure, protein metabolites cannot be excreted by the kidneys. Combined with infections, trauma, or fasting, increased protein catabolism leads to a sharp rise in blood non-protein nitrogen, resulting in azotemia and uremic symptoms. Grade I cases show no significant symptoms. Grade II cases exhibit nausea, vomiting, abdominal distension, diarrhea, and other gastrointestinal symptoms. Severe cases may progress to drowsiness, unconsciousness, or death.
5. Hypertension: About two-thirds of acute renal failure patients develop varying degrees of hypertension, mainly due to excessive pressor substances from renal ischemia.
6. Heart failure: Heart failure is a major complication in the oliguric phase, often occurring after pulmonary edema and hypertension, requiring close attention.
7. Bleeding tendency: Due to platelet dysfunction, increased capillary fragility, and inhibited prothrombin production, acute renal failure can cause significant bleeding tendencies, such as epistaxis, subcutaneous bruising, and oral, gingival, or gastrointestinal bleeding.
8. Anemia: Almost all cases exhibit progressive anemia. Causes include excessive red blood cell loss or destruction from trauma, bleeding, or hemolysis, as well as uremic toxins suppressing bone marrow erythropoiesis.
(III) Diuretic phase
If patients receive proper treatment and safely pass the oliguric phase, the necrotic renal tubular epithelial cells gradually regenerate, and undamaged nephrons recover function, leading to the diuretic phase. The main manifestations include:
1. Polyuria: Increased urine output is the main feature of the polyuric phase, which is caused by the inability of the regenerated renal tubules to concentrate urine, combined with the osmotic diuretic effect of high concentrations of urea retained in the blood, as well as the diuretic effect of retained water, electrolytes, and metabolic products in the body. The speed and extent of the increase in urine output are related to the recovery of the patient's renal function and the body's water content. If the patient had severe edema during the oliguric phase, received excessive fluid intake, and had slow renal function recovery, the urine output during the polyuric phase may suddenly increase significantly. If the patient was already dehydrated during the oliguric phase, the urine output will gradually increase. If there are old sexually transmitted disease lesions in the kidneys, the urine output will increase slowly and plateau at 500–700 milliliters, often indicating a poor prognosis.
2. Water and electrolyte imbalance: Due to excessive urination, if not properly replenished, the patient may experience dehydration. When daily urine output exceeds 1000 milliliters, the renal tubule function is not yet fully developed, leading to the excretion of a large amount of potassium ions in the urine. If replenishment is insufficient, hypokalemia may occur. Additionally, during the polyuria phase, the excretion of a large amount of sodium ions can also lead to sodium-deficient hyponatremia. Attention should be paid to this.
3. Azotemia: In the early stage of the polyuria phase, the blood non-protein nitrogen (NPN) may continue to rise. The reason is that although the filtration and excretion of solutes by the kidneys have increased, they are still insufficient in the short term to clear the accumulated metabolic products in the body. Furthermore, some nitrogenous metabolic products may reabsorb from the renal tubules, exacerbating azotemia. Subsequently, as kidney function continues to recover, the levels of non-protein nitrogen, urea nitrogen, and creatinine in the blood will rapidly decline. The patient's overall condition will begin to improve quickly, with improved mental state and gradually increasing appetite.
(IV) Recovery Period
As kidney function gradually recovers, blood non-protein nitrogen levels return to normal, electrolyte imbalances are corrected, and urine output returns to normal levels, the patient's condition improves day by day. However, due to the physical toll of the disease course, symptoms such as weakness, emaciation, and anemia may persist. The kidney's concentrating ability may not fully recover, and low-specific-gravity urine may continue for several months.
Oliguria and anuria occurring on the basis of diseases caused by surgery, trauma, shock, or hemorrhage are clues to the diagnosis of acute renal failure. If the urine output is less than 17 milliliters per hour or less than 400 milliliters in 24 hours; or if, after anti-shock treatment and adequate blood volume replenishment for more than 3 hours, the urine output remains below 17 milliliters per hour, or even less than 100 milliliters in 24 hours, acute renal failure can be considered to have occurred. Immediate further examination should be conducted for differentiation and definitive diagnosis.
(1) Initial Stage
1. Diagnosis of hypovolemia: ① History of blood loss, shock, dehydration, etc. ② Low or normal blood pressure, narrow pulse pressure, increased pulse rate. ③ Low urine output, but specific gravity above 1.020, normal urinalysis. ④ Central venous pressure below 6 cm H₂O. ⑤ Increased urine output after fluid challenge test.
2. Diagnosis of renal vasospasm: ① After correcting hypovolemia, signs of dehydration and shock disappear, but urine output remains low. ② Urine specific gravity above 1.020, normal urinalysis, or presence of a few hyaline and fine granular casts. ③ No response to fluid challenge test. ④ After intravenous infusion of diuretic mixture, urine output may increase due to relief of renal vasospasm. The composition of the diuretic mixture is as follows:
| Procaine | 1 g |
| Aminophylline | 0.25–0.5 g |
| Sodium benzoate caffeine | 0.25–0.5 g |
| Vitamin C | 1–3 g |
| Papaverine | 30 mg |
| 10–25% Glucose | 200–500 ml |
⑤ Positive mannitol test; after intravenous injection of 20% mannitol 25–50 g, urine output exceeding 40 ml per hour indicates preserved renal tubular function, suggesting prerenal oliguria due to renal vasospasm.
(2) Diagnostic Criteria for Oliguric or Anuric Stage
1. No signs of hypovolemia, blood pressure normal or elevated.
2. Urine output less than 400 ml in 24 hours, or less than 17 ml per hour.
3. Urine specific gravity fixed around 1.010, generally not higher than 1.010.
4. Positive urine protein, urinalysis showing red blood cells, coarse granular casts, large numbers of renal tubular epithelial cells, necrotic epithelial cell casts, and sometimes hemoglobinuria and pigmented casts.
5. Urinary sodium content often exceeds 40 mEq/L, at least not below 30 mEq/L.
6. Rapid and significant rise in serum potassium and non-protein nitrogen.
7. No response to mannitol test.
bubble_chart Treatment Measures
(1) Treatment in the Initial Stage
1. Treatment Based on Disease Causes: Due to the numerous causes of acute renal failure, only the key points will be discussed.
(1) Active prevention and treatment of shock, correcting hypovolemia: For shock caused by various reasons, all measures should be taken to replenish blood volume as quickly as possible, restore blood pressure, and ensure renal blood flow. During anti-shock treatment, the use of vasopressors must be carefully considered. Vasopressors that cause severe renal vasoconstriction, especially norepinephrine, should be avoided.
(2) For hemolytic acute kidney failure, the following measures should be taken: ① Intravenous infusion of sodium bicarbonate solution to alkalinize urine, prevent methemoglobin from blocking renal tubules, and correct metabolic acidosis. ② Intravenous administration of mannitol for osmotic diuresis. ③ Use of hydrocortisone to alleviate antigen-antibody reactions, reduce hemolytic symptoms, and increase renal blood flow. ④ Exchange transfusion therapy may be considered if necessary.
(3) In cases of drug poisoning, residual toxins in the gastrointestinal tract should be promptly eliminated, and antidotes should be administered, such as activated charcoal, milk, egg white water, and dimercaprol.
2. Relieving Renal Vascular Spasm and Improving Renal Blood Circulation
(1) Use of 654-2: 654-2 can relieve microvascular spasm and has antiplatelet aggregation effects, aiding in the improvement of microcirculation. Therefore, high-dose administration of 654-2 plays a significant role in preventing and treating acute renal failure.
(2) Use of vasodilators: Such as aminophylline, papaverine, procaine, sodium benzoate caffeine, phenoxybenzamine, and phentolamine.
3. Use of Diuretics: Osmotic diuretics include mannitol and sorbitol. Potent diuretics include ethacrynic acid and furosemide.
(2) Treatment in the Oliguric Stage
1. Dietary Control: Provide a high-carbohydrate, low-protein diet. Protein intake should be limited to 0.3–0.4 g/kg per day, with high-quality protein containing essential amino acids. Sufficient calories (1000–2000 kcal/day) must also be provided.
2. Fluid Control: The principle of "better slightly less than too much" should be followed for fluid intake. The daily maximum can be calculated using the following methods:
(1) Daily requirement equals visible fluid loss plus invisible fluid loss minus endogenous water production. Generally, endogenous water production in adults is 400 mL, and invisible fluid loss is 800 mL. In practice, 400 mL can be used as the base plus the previous day's urine output and other excretions.
(2) Calculation based on body weight: If daily weight loss is 0.2–0.5 kg without significant changes in serum sodium, fluid replacement is appropriate.
3. Correcting Electrolyte Imbalance: Prevention and Treatment of Hyperkalemia: Patients in this stage are prone to hyperkalemia, which may be asymptomatic early on but can suddenly become fatal in severe cases. Close monitoring and active prevention and treatment are essential.
(1) Use of Calcium: Calcium ions do not lower serum potassium but can counteract the inhibitory effect of potassium on the heart and enhance myocardial contraction. Administer 10% calcium gluconate (50–100 mL) or 5% calcium chloride (50 mL) intravenously in divided doses or as an infusion. Avoid excessive single doses and rapid administration.
(2) Use of Sodium Solution: Sodium is an antagonist of potassium. Lactate or sodium bicarbonate solution is typically used, as it not only counteracts potassium ions but also corrects metabolic acidosis, aiding in the treatment of hyperkalemia.
(3) Use of Hypertonic Glucose and Insulin: Hypertonic glucose and insulin can shift extracellular potassium into cells, alleviating hyperkalemia. The general ratio is 3 g of glucose per 1 unit of insulin.
(4) Sodium/Calcium Polystyrene Sulfonate Resin Enema: Each gram of resin can exchange 3 mEq of potassium. Administering 20–60 g of resin in 150–400 mL of water as a retention enema can remove 60–180 mEq of potassium.
(5) Dialysis Therapy
The treatment of hyponatremia mainly involves fluid restriction and is generally not actively managed.
Calcium should be supplemented when hypocalcemia causes spasm symptoms. Generally, 10% calcium gluconate can be administered intravenously.
For symptoms caused by hypermagnesemia, calcium agents as magnesium antagonists can be used for treatment.
Metabolic acidosis is highly hazardous and should be corrected when severe. Sodium bicarbonate solution or sodium lactate solution is commonly used.
4. Prevention and Treatment of Azotemia and Uremia
(1)Provide sufficient calories: no less than 2000 kcal per day, including over 150 grams of glucose. Protein intake should be controlled.
(2)Use drugs that promote protein anabolism, such as testosterone propionate and nandrolone phenylpropionate.
(3)Chinese medicinals enema: 30g raw Cinnamon Twig, 30g raw Rhubarb Rhizoma, and 30g Sophora Flower decocted with 150–200ml of water for enema every six hours.
(4)If blood urea nitrogen exceeds 100 mg%, dialysis therapy should be applied.
5. Infection Control: Sulfonamides are generally not used during acute kidney failure. Antibiotics such as tetracyclines, streptomycin, kanamycin, and polymyxin are excreted by the kidneys and may cause cumulative toxicity in a short period, so they should be avoided if possible. Generally, ampicillin, carbenicillin, chloramphenicol, erythromycin, and penicillin can be used.
6. Chinese medicine treatment: Clinically, it is necessary to first identify whether the kidney's abnormal debilitation is of yin or yang and treat accordingly. Due to the mutual rooting of yin and yang, yin impairment affects yang, and yang impairment affects yin, pure kidney yang or kidney yin deficiency is rare. It is generally classified into two types: kidney yang deficiency and kidney yin deficiency.
(1)Kidney yin deficiency type
Main symptoms: soreness in the waist and spine, bleeding from the mouth and nose, vexing heat in the chest, palms, and soles, slightly elevated blood pressure, flushed heat, dry stools, red tongue with little or cracked or peeled coating, thin and rapid or wiry pulse.
Treatment principle: Nourish yin and support yang.
Prescription: Modified Six-Ingredient Rehmannia Decoction.
15g roasted Unprocessed Rehmannia Root, 6g cleaned Cornus officinalis, 15g stir-fried Chinese Yam, 6g stir-fried Moutan Bark, 15g Poria, 15g stir-fried Alisma, 9g Lycium fruit, 9g Eucommia Bark.
(2)Kidney yang deficiency type
Main symptoms: Mental fatigue and lack of strength, nausea and vomiting, poor appetite, sallow face and pale lips, drowsiness or unconsciousness, fear of cold and cold limbs, clear urine, frequent nocturia, plump and tender tongue texture, white coating, deep and weak or weak and large pulse.
Treatment principle: Warm kidney yang and nourish kidney yin.
Prescription: Modified Golden Chamber Kidney Qi Decoction. Add 3g cooked Aconite Lateral Root and 2g Cassia Bark to the above formula.
7. Other Treatments
(1)Physical therapy: Diathermy over the kidney area.
(2)Block therapy: Perirenal fat capsule block therapy.
(3)The use of coenzyme A and adenosine triphosphate plays a certain role in promoting kidney repair and functional recovery.
(III)Treatment of Polyuric Phase
When the 24-hour urine output exceeds 400ml, the polyuric phase begins, indicating the start of kidney parenchyma repair. The polyuric phase is divided into two stages: the early stage, from when the 24-hour urine output exceeds 400ml until the blood non-protein nitrogen begins to decline. During this stage, kidney function recovery is relatively poor, solute excretion is low, and water reabsorption is also low, so blood chemistry may not improve and sometimes non-protein nitrogen levels may even rise. Therefore, management is similar to the oliguric phase. The late stage [third stage] begins when non-protein nitrogen starts to decline until it returns to normal. During this stage, the patient's general condition gradually improves, and appetite increases, but due to the significant loss of water and electrolytes, inadequate supplementation can lead to complications. Thus, the main treatments during this phase are:
1. Maintain water balance: Most patients in the oliguric phase are in a state of varying degrees of water overload. As the polyuric phase begins, allow the excess water to be excreted naturally to achieve a new balance. Fluid replacement should be 1/3 to 2/3 of the urine output. Replacing fluids equal to the urine output may prolong the polyuric phase.
2. Maintain electrolyte balance: With the excretion of water, a large amount of electrolytes is inevitably lost, so timely supplementation is necessary. Generally, 500 milliliters of normal saline should be supplemented per liter of urine, and potassium salts should be supplemented as appropriate when the 24-hour urine output exceeds 1500 milliliters.
3. Infection Prevention: Patients in this stage are often very weak, with extremely low resistance, making them highly susceptible to infections. Active prevention and treatment are essential.
4. Nutritional Support: Gradually increase the intake of high-quality proteins. Blood transfusions may be administered for severe anemia.
(4) Treatment During the Recovery Phase
Due to the significant protein imbalance following acute renal failure, the primary treatment approach during this phase is active nutritional supplementation, including a high-protein, high-carbohydrate, and high-vitamin diet. Additionally, physical activity should be gradually increased to promote the recovery of organ functions throughout the body. Full recovery of kidney function often takes more than a year.
(1) Differentiation between acute renal failure and oliguria caused by dehydration is shown in the following table:
Table 1: Differentiation between Acute Renal Failure and Dehydration
| Item | Acute Renal Failure | Dehydration |
| 1. Medical History | Shock, poisoning, trauma, surgery, etc. | Fluid loss, insufficient intake |
| 2. Urine Specific Gravity | Low and fixed around 1.010 | Above 1.020 |
| 3. Urine Routine | Protein +, may have red blood cells and granular casts, etc. | Normal |
| 4. Urine Sodium | Above 40 mEq/L, at least not below 30 mEq/L | Mostly below 15 mEq/L |
| 5. Hematocrit | Normal or decreased | Increased |
| 6. Plasma Protein | Normal or decreased | Increased |
| 7. Blood Sodium | Decreased | Variable |
| 8. Blood Potassium | Rises rapidly | Grade I: Rises or falls |
| 9. Azotemia | Significant | Grade I |
| 10. Central Venous Pressure | Normal or slightly elevated | Below normal |
| 11. Urine-to-Plasma Urea Ratio | <5 | >5 |
| 12. Fluid Challenge Test | No increase in urine output | Increase in urine output |
(2) Differentiation between acute renal failure and postrenal anuria
1. Medical History: Postrenal anuria has no history of shock, trauma, hemolysis, or dehydration. If there is a surgical history, it is often gynecological or pelvic surgery, which differs from acute renal failure. Additionally, postrenal anuria often occurs suddenly, with a 24-hour urine output typically around 50 mL or even complete anuria.
2. Clinical Manifestations: Postrenal anuria often presents with unilateral or bilateral renal distending pain before or after the onset of anuria. Upon palpation, the kidney root of the nose may sometimes be felt, with tenderness or percussion pain. If these signs are limited to one side, the diagnostic significance of postrenal anuria is greater.
3. Laboratory and other examinations: In postrenal anuria, if urine is available for examination, its specific gravity is generally normal, and there are no casts in the urine. If caused by stones or subcutaneous nodes, red blood cells and pus cells may be present in the urine. In postrenal anuria, if cystoscopy and ureteral catheterization are performed, obstruction is often found at a certain segment of the ureter. Sometimes, the catheter can bypass the obstruction and enter the renal pelvis, draining a large amount of urine. In acute renal failure, even if the catheter is inserted into the renal pelvis, it cannot drain a significant amount of urine. On X-ray plain films of the urinary tract in postrenal anuria, the kidney shadow on the obstructed side may be enlarged, and sometimes clues to the primary disease can be found, such as calcification points in the renal subcutaneous node or positive shadows of stones. In isotopic renography for postrenal anuria, the excretion phase shows a continuous rise, presenting an obstructive renogram. In acute renal failure, the kidney excess parenchymal phase is abnormal.
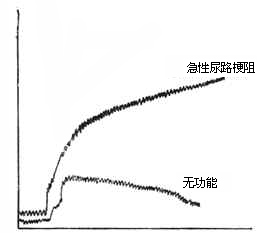
Figure 1 Identification of Isotope Renogram
(III) Differentiation Between Functional Acute Kidney Failure (Prerenal Oliguria) and Organic Acute Kidney Failure (Renal Oliguria)
1. Urinary Sediment Examination: In functional acute kidney failure, only hyaline and fine granular casts are usually present, whereas in organic acute kidney failure, epithelial cell casts, degenerated cell casts, and a large number of coarse granular cell casts appear, along with a significant amount of free renal tubular epithelial cells.
2. Urine-Plasma Osmolality Ratio: In functional acute kidney failure, urine osmolality is normal or elevated (greater than 600 mOsm/L), and the urine-plasma osmolality ratio is greater than 2:1. In organic acute kidney failure, urine osmolality approaches plasma osmolality (300 mOsm/L), and the ratio is less than 1:1.
3. Urinary Sodium Concentration: In functional acute kidney failure, the reabsorption function of sodium is not impaired, so sodium is retained, and the urinary sodium concentration is less than 20 mEq/L. In organic acute kidney failure, sodium reabsorption is reduced, leading to a urinary sodium concentration often exceeding 40 mEq/L.
4. Urine-Plasma Creatinine Ratio: In functional acute kidney failure, the urine concentration function is not impaired, so the urine-plasma creatinine ratio is usually greater than 40:1. In organic acute kidney failure, renal tubular degeneration and necrosis occur, impairing the urine concentration function, and the urine-plasma creatinine ratio is often less than 10:1.
5. Blood Urea Nitrogen-Creatinine Ratio: In functional acute kidney failure, the flow rate in the renal tubules decreases, and the reabsorption of filtered urea increases while creatinine excretion remains constant. Therefore, the blood urea nitrogen-creatinine ratio is greater than 20:1. In organic acute kidney failure, the ratio is typically 10:1.
6. One-Hour Phenolsulfonphthalein Excretion Test: The conventional phenolsulfonphthalein test is performed, but only a one-hour urine specimen is collected. The bladder is flushed with saline to minimize errors caused by residual urine. Phenolsulfonphthalein excretion requires adequate renal blood flow and tubular secretion function. Thus, minimal excretion often indicates organic acute kidney failure. If phenolsulfonphthalein excretion exceeds 5%, functional acute kidney failure may be present, with tubular function not fully impaired.
Table 2 Diagnostic Indicators of Functional and Organic Acute Kidney Failure
| Functional | Partially Organic | Fully Organic | |
| 1. Urine-Plasma Osmolality Ratio | >2:1 | 1.9:1–1.1:1 | <1.1:1 |
| 2. Urinary Sodium Concentration | <20毫當量/升 | 20–40 mEq/L | >40 mEq/L |
| 3. Urine-Plasma Creatinine Ratio | >40:1 | 40:1–10:1 | <10:1 |
| 4. Blood Urea Nitrogen-Creatinine Ratio | >20:1 | 40:1–10:1 | <10: 1 |
| 5. One-hour phenol red excretion rate | >50% | 1~5% | 0~trace |






