| disease | Cardiospasm |
| alias | Achalasia, Megaesophagus |
Cardiospasm, also known as achalasia, is a common disorder of esophageal motility. The normal peristalsis of the esophagus disappears, and the lower esophageal sphincter fails to relax during swallowing, causing food to stagnate in the esophagus. In long-standing cases, the upper and middle segments of the esophagus become significantly dilated, accompanied by varying degrees of dysphagia.
bubble_chart Epidemiology
Cardiospasm may lead to epiphrenic esophageal diverticulum and carcinogenesis based on chronic inflammation of the esophageal mucosa and increased luminal pressure. Statistics show that the incidence of esophageal cancer in long-standing cases of cardiospasm can reach 2-7%.
The disease cause of achalasia is not yet fully understood. Currently, it is mostly believed to be caused by dysfunction of esophageal motor nerves. Histomorphological examination of esophageal tissue reveals degeneration, reduction, or absence of ganglion cells in the Auerbach's plexus of the muscular layer in the thoracic esophagus. Due to defects in parasympathetic innervation, the esophageal wall exhibits low tension, loss of peristalsis, and spasm of the lower esophageal sphincter. The cause of the esophageal nerve lesions remains inconclusive. Postmortem pathological examinations suggest that the primary lesion may be located in the vagus nerve or its central nuclei. Animal experiments have also shown that damaging the motor nuclei of the vagus nerve in dogs and cats can produce similar esophageal lesions.
bubble_chart Pathological Changes
The lower segment of the esophagus and the cardia exhibit hypertrophy of the muscular layer, leading to narrowing of the esophageal lumen. Due to food retention in the esophageal cavity, the esophageal mucosa becomes thickened, edematous, inflamed, and may even develop erosions or ulcers. In cases with a prolonged course, the middle and upper segments of the esophagus become significantly dilated. In severe cases, the esophagus elongates and becomes tortuous, resembling the shape of the sigmoid colon.
bubble_chart Clinical Manifestations
Cardiospasm is more common in young and middle-aged adults, with a slow onset and a prolonged course. The most common early symptoms are dysphagia, retrosternal pain, and a sensation of obstruction. Sometimes solid foods are easier to swallow than liquids, and warm foods are easier to swallow than cold ones. The symptom of dysphagia has a {|###|}stage of remission{|###|}, occurring intermittently with varying severity. Emotional changes can influence the symptoms, which worsen during periods of stress or agitation. These characteristics can help differentiate it from dysphagia caused by {|###|}esophagus cancer{|###|} or esophageal scar stenosis. As the disease progresses, the {|###|}stage of remission{|###|} gradually disappears, and dysphagia becomes persistent. Due to food retention in the esophagus, {|###|}vomiting{|###|} often occurs, with undigested food and no gastric acid. If food remains in the esophagus for a long time before being regurgitated, it may have a foul odor. Overflow of food into the respiratory tract can lead to aspiration pneumonia or lung abscess. Complications such as esophagitis or {|###|}mucous membrane ulcer{|###|} can exacerbate retrosternal pain and may cause slight {|###|}hematemesis{|###|}. In cases with a long course and severe dysphagia, weight loss may occur.
Physical examination generally reveals no abnormalities.
Barium swallow X-ray examination shows barium retention at the cardia, with a smoothly tapered, bird-beak-like narrowing in the lower esophagus. The barium flows slowly into the stomach in a thin stream. The middle and lower segments of the esophageal lumen are dilated, and in severe cases, the esophageal lumen becomes significantly thickened, elongated, and tortuous, forming an "S" shape resembling the sigmoid colon. Normal peristalsis of the esophageal wall is weakened or absent, and sometimes irregular weak contractions may occur. This helps differentiate it from cicatricial stenosis and esophagus cancer (Figure 1).
| | | |
| Esophageal cicatricial stenosis | Cardiospasm | Esophagus cancer |
Figure 1 Barium swallow images of common esophageal diseases
Esophagoscopy can confirm the diagnosis and exclude esophageal cicatricial stenosis and esophageal tumors. In patients with cardiospasm, the esophageal lumen is dilated with retained food and mucosal edema and inflammation. The lower esophagus shows persistent spasm with a narrowed lumen, but the mucosa remains intact without scar tissue or tumors.
Esophageal manometry aids in definitive diagnosis. The pressure in the high-pressure zone of the lower esophagus is often more than twice that of normal individuals, and the pressure in the lower esophagus and sphincter does not decrease during swallowing. The pressure in the middle and upper esophageal lumen is also higher than normal, and normal peristaltic waves are absent during swallowing. Subcutaneous injection of methacholine chloride (5–10 mg) may enhance esophageal contractions in some cases, significantly increasing the pressure in the middle and upper esophageal lumen and causing severe retrosternal pain (Figure 2).
Figure 2 Esophageal pressure curves in healthy individuals and patients with cardiospasm
bubble_chart Treatment Measures
(1) Drug Therapy For early-stage patients with achalasia, the condition should be explained, emotions stabilized, and a diet of small, frequent meals with thorough chewing is recommended. Additionally, sedative and antispasmodic medications can be administered, such as oral 1% procaine solution, sublingual nitroglycerin tablets, or the more recently tested calcium antagonist nifedipine, which can alleviate symptoms. To prevent food from refluxing into the respiratory tract during sleep, a high pillow or elevated bed head can be used, and esophageal lavage before sleep may be necessary if required.
(2) Lower Esophageal Dilation A catheter with a balloon at its tip is inserted into the cardia, and the balloon is inflated with water, barium, or mercury to dilate it. The catheter is then forcefully pulled out to rupture the muscle fibers, thereby widening the narrowed lumen of the lower esophagus. Approximately two-thirds of patients respond well to this treatment, but repeated dilations are often necessary. A minority of patients may face the risk of esophageal perforation. Currently, lower esophageal dilation is only suitable for early-stage cases where surgery is contraindicated or refused and the esophagus has not yet become severely dilated.(3) Surgical Treatment Various surgical methods have been used to treat achalasia. For example, in 1910, Wendel made a full-thickness longitudinal incision at the cardia and sutured it transversely to enlarge the lumen. In 1912, Heyrovsky performed an anastomosis between the lateral wall of the lower esophagus and the opposite side of the gastric fundus. In 1916, Gröndahl made a horseshoe-shaped incision on the anterior wall of the lower esophagus, cardia, and gastric fundus, then sutured the anterior and posterior walls of the incision to create a long longitudinal new passage between the lower esophagus and gastric fundus. These procedures effectively relieved cardia obstruction, allowing food to pass smoothly from the esophagus into the stomach. However, because they altered the anatomical relationship of the cardia and destroyed the lower esophageal sphincter mechanism, gastric acid reflux into the esophagus became highly likely, leading to severe postoperative peptic esophagitis and ulcers. Consequently, these surgeries have been abandoned. In recent years, the surgical procedure with good efficacy and fewer complications for treating achalasia is the longitudinal myotomy of the cardia mucosa, first advocated by Heller in 1913. Initially, the Heller procedure involved bilateral longitudinal myotomy of the esophagus and cardia. Since 1918, improvements have led to the practice of performing the myotomy on only one side. The surgery involves a left posterolateral thoracotomy through the 7th intercostal space or after resection of the 7th rib. The mediastinal pleura is incised, and the distal esophagus is freed without opening the esophageal hiatus, while the vagus nerve is carefully preserved. The lower esophagus and cardia are pulled upward, and a longitudinal incision is made on the left anterolateral wall of the esophagus, extending deep to the mucosa but not penetrating it. The lower end of the incision extends slightly beyond the gastroesophageal junction, with the gastric wall myotomy limited to a few millimeters to 1 cm. The upper end of the incision should reach above the dilated and thickened segment of the esophageal wall, with the length varying according to the condition, typically around 5–10 cm. After completely severing the longitudinal and circular muscle fibers of the esophageal wall, the muscle fibers are carefully dissected between the muscle layer and the mucosa to a width of about half the esophageal circumference. This prevents scar tissue formation between the cut ends of the muscle fibers. Once the muscle fibers are freed, the mucosa bulges out through the myotomy incision, and the mediastinal pleura is sutured (Figure 3).

(1) Exposure of the surgical field
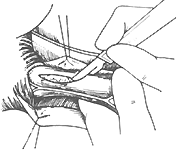
(2) Incision of the muscle layer
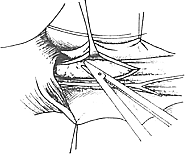
(3) Mobilization of the incised muscle layer
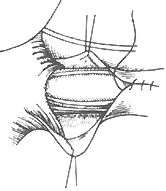
(4) Suture of the mediastinal pleura
Figure 3 Esophageal myotomy
The Heller procedure is simple to perform, has excellent efficacy, and very few complications. Postoperatively, symptoms improve in 90–95% of cases, with a reflux esophagitis complication rate of only 2%.







